Anti-CD43 Glycopeptide Antibody Development Service
Creative Biolabs stands at the forefront of the rapidly evolving field of glyco-immunology, offering a comprehensive and specialized suite of services, including our premier anti-glycopeptide antibody development platform, dedicated to the development of high-affinity antibodies against complex carbohydrate and glycopeptide antigens. We are proud to introduce our premier service for the development of anti-CD43 glycopeptide antibodies. This service is meticulously designed to target tumor-associated glycoforms of CD43 (also known as leukosialin or sialophorin), distinguishing them from the physiological forms ubiquitously found on normal hematopoietic cells. By focusing on aberrant glycosylation patterns, such as the exposure of Tn, T, and sTn antigens on the CD43 peptide backbone, we empower researchers to explore novel diagnostic and therapeutic avenues with unprecedented specificity and reduced off-target toxicity.
CD43 is a major type I transmembrane sialoglycoprotein expressed on the surface of most hematopoietic lineage cells, including T lymphocytes, monocytes, granulocytes, and some B lymphocytes. In its normal physiological state, CD43 is heavily O-glycosylated with complex, branched core 2 oligosaccharides. These extensive glycan structures impart a rigid, rod-like structure to the extracellular domain, extending approximately 45 nm from the cell surface. This structural feature plays a pivotal role in immune cell activation, adhesion, and anti-adhesive functions, effectively providing a "glycocalyx" barrier that modulates cell-cell interactions. However, the enzymatic machinery governing glycosylation is frequently dysregulated in malignant transformation, leading to the expression of truncated, tumor-associated glycoforms. These "neo-glycopeptide" epitopes represent a unique class of tumor-specific antigens (TSAs) that are virtually absent in healthy tissues, making them ideal targets for precision oncology and immunotherapy.
Background: The CD43 Glyco-Code in Cancer
The structural integrity and biological function of CD43 are intrinsically linked to its O-glycosylation status. The extracellular domain of human CD43 contains approximately 80 serine and threonine residues that are potential sites for O-linked glycosylation. In healthy resting lymphocytes and myeloid cells, these sites are predominantly occupied by complex core 2 branched O-glycans (Galβ1-3(GlcNAcβ1-6)GalNAcα-Ser/Thr). The biosynthesis of these structures is strictly controlled by the enzyme core 2 β-1,6-N-acetylglucosaminyltransferase (C2GnT), which initiates the branching of the O-glycan chain. The presence of these bulky, negatively charged glycans prevents non-specific cell-cell interactions and protects the polypeptide backbone from proteolytic cleavage, maintaining immune homeostasis.
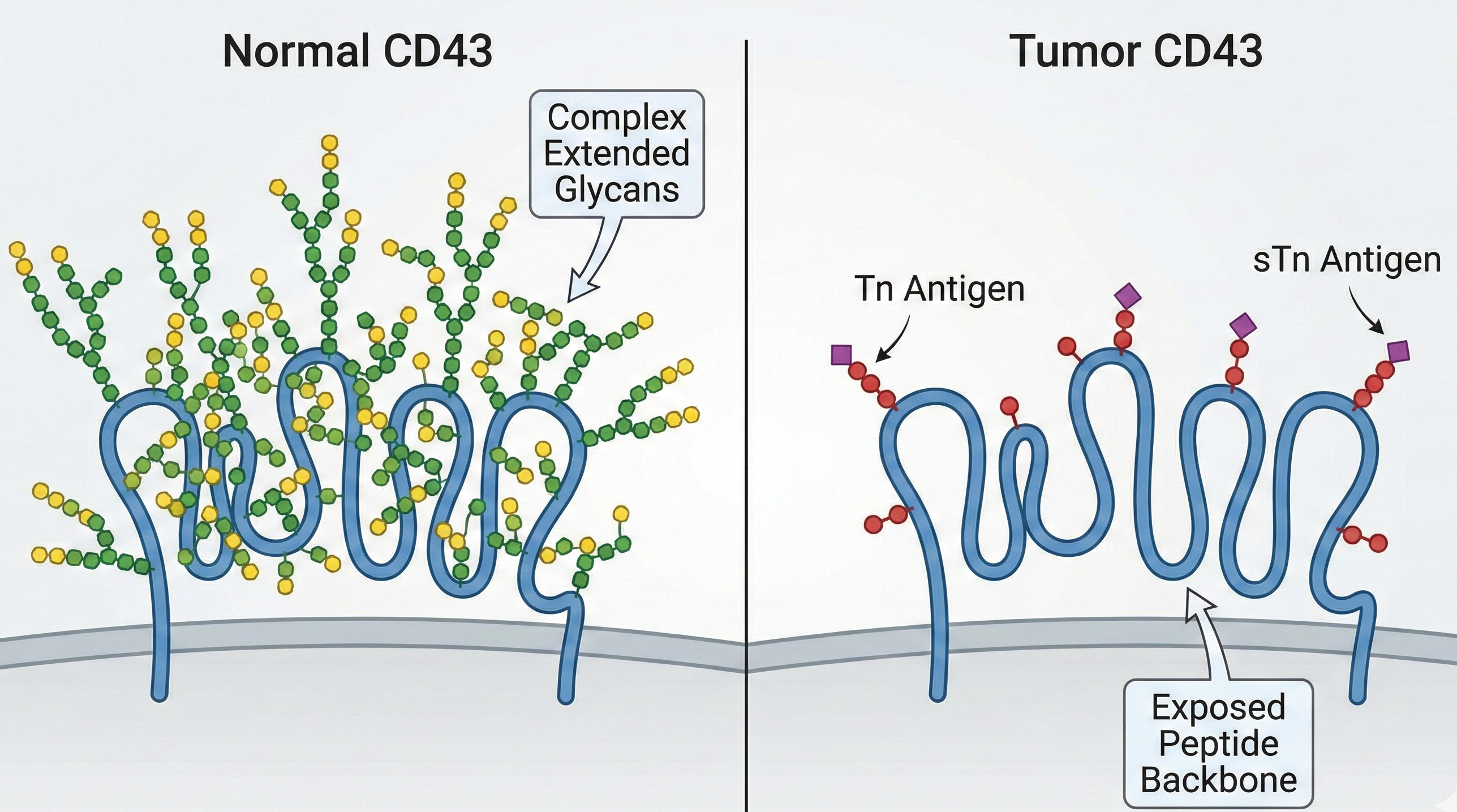 Fig.1 Schematic representation of CD43 glycosylation changes in cancer.
Fig.1 Schematic representation of CD43 glycosylation changes in cancer.
In contrast, malignant cells often exhibit a profound alteration in their glycosylation profile, a phenomenon widely recognized as a hallmark of cancer. In many carcinomas and hematological malignancies, there is a significant downregulation or loss of C2GnT activity, often accompanied by the upregulation of specific sialyltransferases (e.g., ST6GalNAc). This metabolic shift results in the premature termination of glycan chain elongation, leading to the expression of simple, truncated carbohydrate antigens such as the Tn antigen (GalNAcα-Ser/Thr), the Thomsen-Friedenreich (T) antigen (Galβ1-3GalNAcα-Ser/Thr), and the Sialyl-Tn (sTn) antigen (NeuAcα2-6GalNAcα-Ser/Thr) directly on the CD43 peptide backbone. These truncated glycans expose the underlying peptide sequence, creating novel "glycopeptide" epitopes that are immunologically distinct from the fully glycosylated CD43 found on normal cells.
This phenomenon, often referred to as "aberrant glycosylation," generates highly specific tumor markers. For instance, the UN1 monoclonal antibody recognizes a unique epitope formed by the combination of a specific peptide sequence and a truncated saccharide chain on CD43, which is expressed in T-cell leukemias and various solid tumors but not in normal tissues. Targeting these combinatorial epitopes offers a significant advantage over targeting purely protein or purely carbohydrate antigens, as it combines the specificity of the peptide sequence with the tumor-restricted expression of the aberrant glycan, thereby enhancing the therapeutic index and reducing the risk of autoimmune adverse events.
Pain Points: Challenges in Targeting CD43
Despite the attractive therapeutic potential of CD43 as a target, the development of therapeutic antibodies against this molecule has been historically fraught with significant scientific and technical challenges.
Homology with Normal Tissue
The primary obstacle lies in the ubiquitous expression of normal CD43 on the surface of almost all leukocytes. Standard "pan-CD43" antibodies inevitably cross-react with healthy immune cells, leading to potential on-target/off-tumor toxicities like lymphopenia.
Low Immunogenicity
Carbohydrate antigens are generally T-cell independent with poor immunogenicity. They fail to recruit T-cell help, often eliciting low-affinity IgM responses rather than the robust, high-affinity IgG responses required for therapeutic efficacy.
Tolerance Mechanisms
The immune system is often tolerant to self-antigens, making it difficult to break tolerance against the peptide backbone of CD43, which is a self-protein. Conventional immunization strategies frequently fail to distinguish between glycoforms.
Undefined Specificity
Many commercially available antibodies have poorly defined specificities. For clinical success, it is imperative to generate antibodies validated to bind exclusively to the neo-glycopeptide interface, ensuring minimal cross-reactivity.
Our Comprehensive Development Solutions
To address these multifaceted challenges, Creative Biolabs has developed a specialized, integrated platform for the generation of tumor-specific anti-CD43 glycopeptide antibodies. Our approach leverages decades of experience in carbohydrate chemistry and antibody engineering to offer a seamless workflow from immunogen design to lead candidate validation.
Rational Immunogen Design & Synthesis
We utilize advanced solid-phase peptide synthesis (SPPS) and chemo-enzymatic glycosylation techniques to create defined glycopeptide immunogens. By precisely positioning Tn, sTn, or T antigens on specific serine and threonine residues within the CD43 sequence, we engineer immunogens that structurally mimic the tumor-associated epitope with atomic-level precision. These synthetic glycopeptides are then conjugated to highly immunogenic carrier proteins (such as KLH, DT, or BSA) using optimized linker chemistries to enhance T-cell dependent immune responses and break tolerance.
Advanced Discovery Platforms
We offer a multi-pronged discovery approach tailored to the specific needs of the project. Our proprietary hybridoma technology utilizes specialized transgenic mouse strains engineered to produce human antibodies or to have an expanded immune repertoire, facilitating the generation of high-affinity IgG antibodies. Alternatively, our phage display platform allows for the high-throughput screening of vast immune or synthetic libraries (human, Llama VHH, rabbit) to isolate binders with unique paratope geometries capable of recognizing deep glycopeptide clefts that are often inaccessible to conventional antibodies.
Dual-Flow Screening Strategy
Specificity is the cornerstone of our selection process. We employ a rigorous "positive/negative" dual-flow screening strategy. Clones are selected for strong binding to the target glycopeptide via ELISA and surface plasmon resonance (SPR). Crucially, they are simultaneously counter-screened against the naked peptide, irrelevant glycopeptides, and fully glycosylated CD43 derived from normal donor leukocytes. This ensures the selection of antibodies that are strictly dependent on the specific aberrant glycan-peptide interface and lack cross-reactivity with healthy tissues.
Functional & Biophysical Characterization
Lead candidates undergo extensive characterization to ensure their suitability for downstream applications. We perform detailed kinetic analysis to determine binding affinity (KD), on-rates, and off-rates. Specificity is further validated using high-density glycan arrays and flow cytometry on a panel of antigen-positive tumor cell lines versus antigen-negative normal PBMCs. We also offer assays to assess suitability for Antibody-Drug Conjugate (ADC) development and fine epitope mapping services to pinpoint the exact binding residues.
Project Workflow
Request a Quote for Anti-CD43 Antibody Services
Highlights and Core Advantages
Exquisite Specificity
Our screening protocols guarantee minimal to no cross-reactivity with normal physiological CD43.
Versatile Formats
Development of full-length IgG, scFv, Fab, and VHH tailored to your application.
Deep Expertise
Over a decade of experience in glyco-immunology and challenging antigen development.
Intellectual Property
Full ownership of antibody sequences, hybridoma clones, and all associated data is transferred to the client.
Applications in Research
- Antibody-Drug Conjugates (ADCs): CD43 is rapidly internalized upon antibody binding in many tumor types. We select for internalizing clones that can effectively deliver cytotoxic payloads to the lysosome, maximizing the therapeutic index of ADC constructs.
- Chimeric Antigen Receptors (CAR-T): The tumor-specificity of our glycopeptide antibodies makes them ideal candidates for scFv generation in CAR-T therapy. Targeting the aberrant glycoform minimizes the risk of "on-target/off-tumor" toxicity against normal hematopoietic cells, a major safety concern in leukemia treatment.
- Bispecific T-cell Engagers: We develop antibodies that can be incorporated into bispecific formats, redirecting cytotoxic T-cells to kill tumor cells expressing the CD43 neo-epitope.
- Diagnostic Immunohistochemistry (IHC): Our high-affinity antibodies serve as robust tools for the detection of aberrant CD43 expression in tissue microarrays, aiding in the stratification of patients and the study of tumor progression.
Request a Quote
Published Data
The therapeutic relevance of targeting specific CD43 glycoforms has been robustly validated in peer-reviewed literature. A seminal study provides compelling evidence for the existence of leukemia-specific CD43 epitopes that can be exploited for targeted therapy. The study investigated the role of CD43 glycosylation in the immune evasion mechanisms of leukemia cells and characterized two monoclonal antibodies, R54 and B2, which were identified to specifically recognize CD43 on MLL/AF9 leukemia cells while sparing normal hematopoietic cells.
The researchers demonstrated that the binding of mAbs R54 and B2 was strictly dependent on the specific glycosylation status of the CD43 molecule. Unlike standard pan-CD43 antibodies (such as S11), which recognize the protein core regardless of glycosylation, R54 and B2 exhibited a distinct binding profile that was sensitive to sialidase treatment, indicating that sialic acid residues are critical components of their epitope recognition. Fig.2 illustrates the specific recognition of CD43 by these antibodies using flow cytometry. The data clearly show that R54 and B2 could effectively distinguish the leukemia-associated CD43 glycoforms from those present on wild-type splenocytes. This high degree of specificity highlights the feasibility of generating antibodies that target the unique "glyco-code" of cancer cells, thereby minimizing off-target effects on normal tissues. This research highlights the scientific potential of developing glycopeptide-specific antibodies for applications in precision medicine.
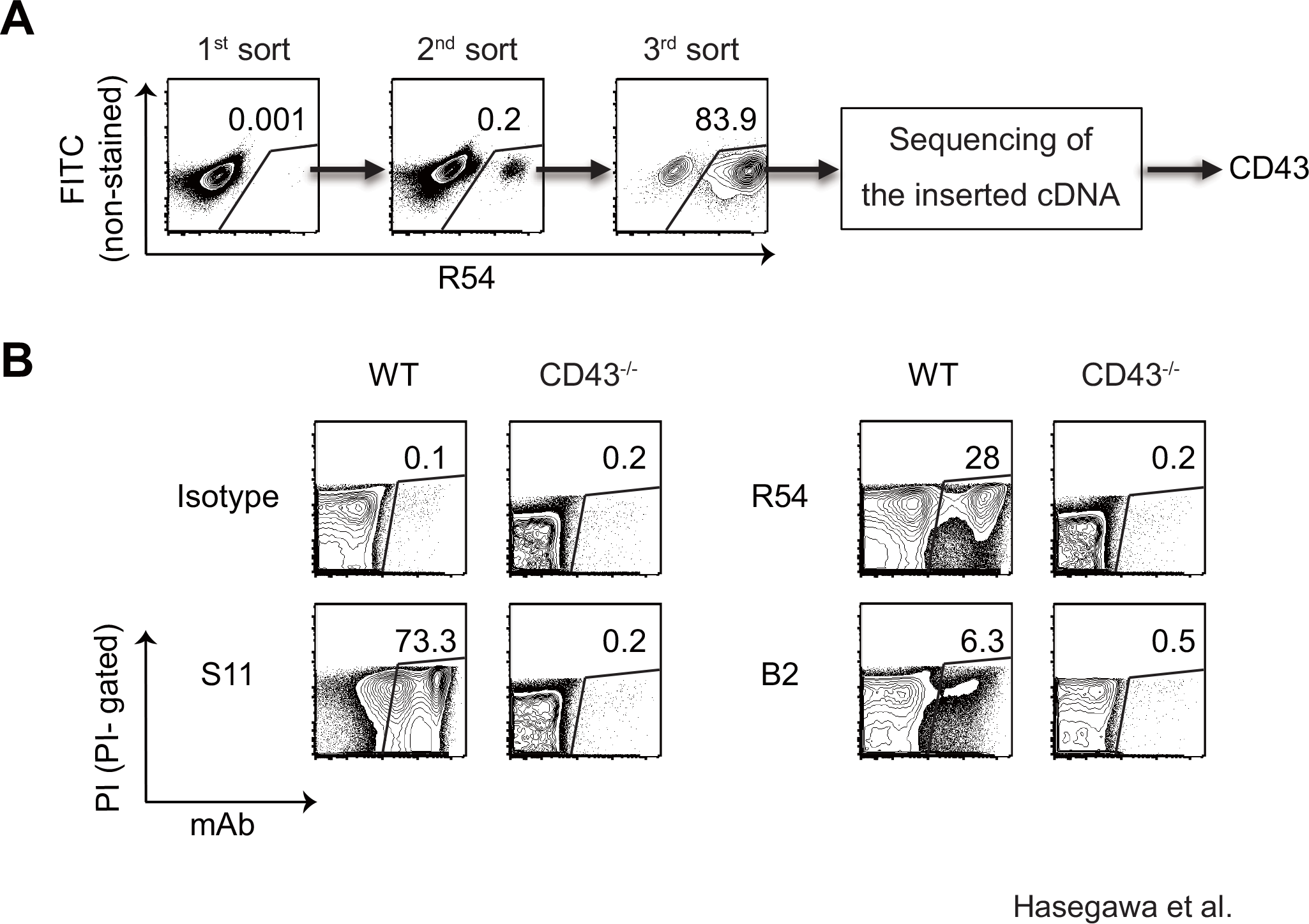 Fig.2 Specific recognition of CD43 by R54 and B2 mAbs.1
Fig.2 Specific recognition of CD43 by R54 and B2 mAbs.1
FAQs
How do you validate the specificity of the antibody for the glycopeptide versus the naked peptide?
We employ a multi-step validation process. Initial screening involves ELISA against the immunizing glycopeptide, the non-glycosylated peptide, and irrelevant glycopeptides. This is followed by flow cytometry using cell lines that express the relevant glycosyltransferases versus wild-type cells. Finally, we use glycan arrays to profile the antibody against hundreds of defined glycan structures to ensure no off-target carbohydrate binding occurs.
Can you humanize the resulting antibodies for downstream therapeutic development?
Yes, absolutely. We offer comprehensive antibody humanization services (CDR grafting and back-mutation) for murine antibodies derived from hybridoma technology. Furthermore, we can perform affinity maturation to ensure that the humanized variants retain or exceed the binding affinity of the parental antibody. For phage display projects, we can screen directly from human synthetic libraries to obtain fully human antibodies from the outset.
What is the typical timeline for a custom glycopeptide antibody project?
The timeline varies depending on the complexity of the antigen and the chosen platform. Generally, a hybridoma project takes approximately 4-6 months from immunogen synthesis to the delivery of purified antibodies. Phage display campaigns can be faster, typically ranging from 3-4 months. We provide a detailed Gantt chart upon project initiation and regular progress updates to ensure transparency and adherence to timelines.
Do you provide the specific peptide sequences used for immunization?
Yes, as a service provider, we operate on a fee-for-service basis. Clients retain full ownership of all intellectual property, including the sequences of the synthesized immunogens, the antibody sequences (DNA and amino acid), and the hybridoma cell lines. We do not claim any royalty rights on the products developed through this service.
Is this service suitable for clinical diagnostic development?
Our services are primarily for research use only (RUO). However, the antibodies we develop are manufactured under strict quality control standards and are characterized to a level that supports their transition into preclinical and clinical development pipelines. We can support the early-stage discovery and validation phases required for developing companion diagnostics or therapeutic candidates.
Reference:
- Hasegawa, K., et al. "Glycosylation Status of CD43 Protein Is Associated with Resistance of Leukemia Cells to CTL-Mediated Cytolysis." PLOS ONE 11.3 (2016): e0152326. Distributed under Open Access license CC BY 4.0. https://doi.org/10.1371/journal.pone.0152326
Supports
- Anti-MUC1 Glycopeptide Antibody Development
- Anti-MUC4 Glycopeptide Antibody Development
- Anti-MUC16 (CA-125) Glycopeptide Antibody Development
- Anti-MUC5AC Glycopeptide Antibody Development
- Anti-MUC2 Glycopeptide Antibody Development
- Anti-Podocalyxin (PODXL) Glycopeptide Antibody Development
- Anti-CD43 Glycopeptide Antibody Development
- Custom Glycopeptide Target Antibody Development
