Dermatan Sulfate in Wound Healing and Fibrosis
Glycosaminoglycans (GAGs) are dynamic architects of the extracellular matrix (ECM), orchestrating cellular behavior through intricate structural interactions. Among them, dermatan sulfate (DS) stands out as a critical regulator of tissue assembly and repair. At Creative Biolabs, we empower researchers to decode these complex biological events through our comprehensive Anti-Dermatan Sulfate (DS) Antibody Development services, providing the precision tools needed to investigate matrix biology and pathological fibrosis.
The Structural Identity of Dermatan Sulfate
Dermatan sulfate, historically known as chondroitin sulfate B, is a specialized sulfated glycosaminoglycan found ubiquitously in the skin, cardiovascular vessels, and tendons. Unlike chondroitin sulfate, which contains D-glucuronic acid (GlcA), DS is distinguished by the presence of L-iduronic acid (IdoA) formed through the epimerization of GlcA residues. This unique sugar backbone confers exceptional conformational flexibility to the polymer chain.
This structural pliability is not merely a biochemical curiosity; it is the foundation of dermatan sulfate function. The IdoA residues allow DS chains to interact specifically with a wide array of proteins, including growth factors, chemokines, and structural collagens. In the context of the ECM, DS is primarily found covalently attached to core proteins such as decorin and biglycan, forming small leucine-rich proteoglycans (SLRPs). These proteoglycans are pivotal in regulating the spacing and stability of collagen fibrils, thereby defining the mechanical properties of connective tissues.
Physiological Mechanisms in Wound Repair
Wound healing is a tightly coordinated biological symphony involving inflammation, proliferation, and remodeling. DS-containing proteoglycans are active participants in each phase, serving as reservoirs for signaling molecules and structural templates for new tissue.
Inflammation and Signaling Modulation
Upon tissue injury, the extracellular matrix is degraded, releasing soluble DS chains. These fragments act as danger-associated molecular patterns (DAMPs), influencing the immune response. Furthermore, the wound healing mechanism relies heavily on the bioavailability of growth factors. DS chains bind with high affinity to heparin-binding growth factors such as fibroblast growth factor-2 (FGF-2) and hepatocyte growth factor (HGF). By sequestering these factors in the matrix or presenting them to their receptors, DS fine-tunes the proliferative signals required for re-epithelialization and granulation tissue formation.
Collagen Interaction and Matrix Assembly
The remodeling phase is characterized by the deposition and organization of collagen. Here, the collagen interaction mediated by DS is indispensable. Decorin, a DS-proteoglycan, binds to specific bands on collagen fibrils. The DS chains form antiparallel bridges between adjacent fibrils, effectively acting as a "molecular ruler" that dictates fibril diameter and spacing. Without functional DS, collagen organization becomes chaotic, leading to structurally weak tissue prone to dehiscence. This interaction is strictly dependent on the iduronic acid content and sulfation pattern of the DS chain.
Dysregulation in Tissue Fibrosis
While DS is essential for normal healing, its dysregulation is a hallmark of pathological fibrosis. Fibrotic diseases, such as systemic sclerosis, liver cirrhosis, and hypertrophic scarring, are characterized by the excessive accumulation of ECM components that disrupt organ architecture.
Research indicates a distinct shift in the sulfation profile and chain length of dermatan sulfate in fibrotic tissues. For instance, in hypertrophic scars, the expression of decorin is reduced, while the fine structure of the associated DS chains is altered, affecting their ability to regulate collagen fibrillogenesis. This leads to the formation of thick, disorganized collagen bundles typical of scar tissue. Furthermore, aberrant DS signaling can sustain chronic inflammation and fibroblast activation, perpetuating the fibrotic cycle. Understanding the specific structural changes in DS during tissue fibrosis is crucial for developing targeted therapeutic interventions that can normalize matrix assembly without compromising tissue integrity.
Key Biological Roles of Dermatan Sulfate
ECM Architecture Regulator
DS-proteoglycans act as molecular bridges, strictly regulating collagen fibril spacing and tissue tensile strength during repair.
Growth Factor Reservoir
DS chains sequester and release critical growth factors (FGF-2, HGF), spatially coordinating inflammation and cell proliferation.
Fibrosis Indicator
Pathological scarring is marked by specific alterations in DS sulfation patterns and chain length, serving as a distinct molecular signature.
Iduronic Acid Plasticity
The unique conformational flexibility of L-iduronic acid allows DS to interact specifically with a diverse range of heparin-binding proteins.
Our Solutions for DS Research
Creative Biolabs provides a suite of advanced services designed to overcome the challenges associated with studying complex carbohydrates. We support academic and industrial researchers in dissecting the roles of DS in health and disease.
Anti-Dermatan Sulfate Antibody Development
We generate high-affinity antibodies capable of distinguishing DS from chondroitin sulfate. Our development platform utilizes optimized immunogens and phage display technology to isolate clones that recognize specific sulfation motifs or the distinct iduronic acid backbone. These antibodies are essential for immunohistochemistry, flow cytometry, and ELISA applications in fibrosis research.
Glycosylation Analysis Services
Characterizing the fine structure of GAGs requires sophisticated analytics. We offer comprehensive profiling services including disaccharide analysis by HPLC/MS, determination of sulfation patterns, and chain length measurements. This service helps quantify changes in DS composition across different stages of wound healing or fibrotic progression.
Custom Glycosylation of Biomolecules
For functional studies, we provide custom synthesis and modification of DS chains and proteoglycans. Whether you need defined oligosaccharides for binding assays or biotinylated DS probes for interaction studies, our chemical biology team delivers reagents tailored to your experimental needs.
Glycoarray Platforms
Screen for DS-binding proteins or characterize antibody specificity using our high-throughput glycoarray platforms. We can array diverse GAG structures to map the "sulfation code" recognized by growth factors, cytokines, and matrix proteins involved in tissue repair.
Inquire About DS Research Tools
Published Data
Effective tissue repair relies on a highly choreographed sequence of molecular interactions within the extracellular matrix (ECM), where proteoglycans (PGs) and glycosaminoglycans (GAGs) serve as critical control nodes. Comprehensive analysis reveals that the transition from the inflammatory phase to tissue remodeling is strictly governed by the balance of these matrix macromolecules. Of particular significance are the small leucine-rich proteoglycans (SLRPs), such as decorin, which frequently carry dermatan sulfate chains. These molecules are instrumental in regulating collagen fibril assembly, ensuring the structural integrity of the newly formed dermis. Crucially, they act as natural antagonists to profibrotic signaling by sequestering growth factors like transforming growth factor-beta (TGF-β). The reviewed data highlights that fibrosis is not merely an excess of collagen but a failure in this ECM-mediated regulation. Specifically, aberrant signaling involving hyaluronan fragments and the loss of suppressive proteoglycan interactions can drive the sustained activation of myofibroblasts. This dysregulation locks the tissue in a perpetual cycle of matrix deposition, leading to hypertrophic scarring and organ dysfunction. Targeting these specific ECM-receptor pathways offers a strategic mechanism to redirect the healing process from pathological fibrosis toward regenerative repair.
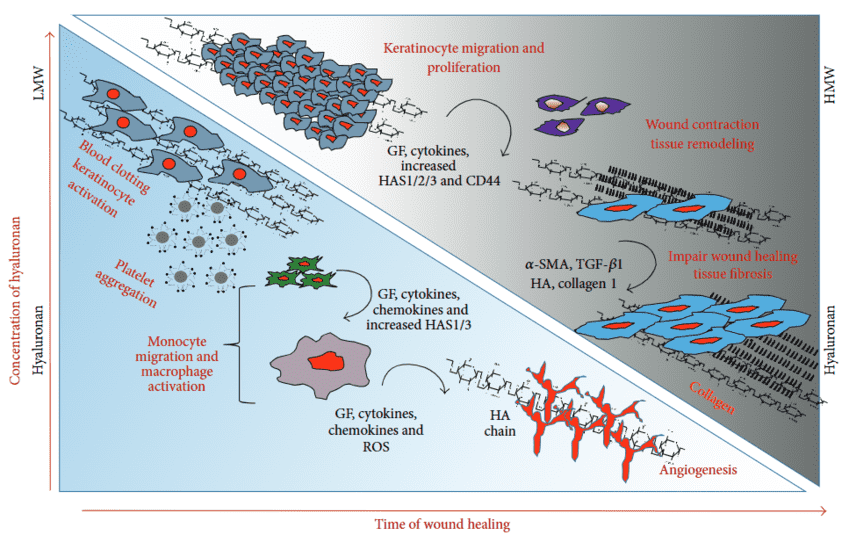 Fig.1
Schematic representation of molecular and cellular transitions distinguishing normal wound repair from pathological fibrosis.1
Fig.1
Schematic representation of molecular and cellular transitions distinguishing normal wound repair from pathological fibrosis.1
FAQs
How does Creative Biolabs distinguish between dermatan sulfate and chondroitin sulfate in antibody development?
We utilize highly specific screening protocols that target the unique conformational epitopes created by L-iduronic acid residues in DS. By using counter-screening against chondroitin sulfate (which contains D-glucuronic acid), we ensure our antibodies possess high specificity for dermatan sulfate motifs.
Can your services analyze the iduronic acid content in tissue samples?
Yes, our Glycosylation Analysis platform employs advanced enzymatic digestion followed by HPLC or Mass Spectrometry to quantify the ratio of IdoA to GlcA, providing a detailed compositional profile of the GAGs in your sample.
What is the significance of investigating DS in fibrosis models?
In fibrotic tissues, the structure of DS often changes, affecting its ability to regulate collagen fibrillogenesis. Studying these alterations can reveal new biomarkers for disease progression and potential targets for anti-fibrotic therapies.
Do you offer reagents for staining DS in histological sections?
We provide a range of anti-DS antibodies validated for immunohistochemistry (IHC) and immunofluorescence (IF). These reagents are optimized to detect DS proteoglycans within the complex environment of the extracellular matrix.
Are your products suitable for clinical diagnostic use?
No, all products and services provided by Creative Biolabs are strictly for research use only (RUO) and are not intended for use in diagnostic or therapeutic procedures in humans.
Supports
- Glycoarray Platforms
- Glycosylation Analysis
- Custom Glycosylation of Biomolecules
- Glycan Science Overview
Reference:
- Ghatak, S.; et al. Roles of Proteoglycans and Glycosaminoglycans in Wound Healing and Fibrosis. Int. J. Cell Biol. 2015, 2015, 834893. Distributed under Open Access license CC BY 4.0. https://doi.org/10.1155/2015/834893
