Bacterial Cell Wall Glycans: Endotoxins and Virulence Factors
The bacterial cell envelope is a complex, multilayered defense system that not only protects the organism from environmental stress but also serves as the primary interface for host-pathogen interactions. Among the diverse array of molecules displayed on the bacterial surface, glycoconjugates—specifically bacterial cell wall components like Lipopolysaccharides (LPS) and Lipoteichoic Acids (LTA)—play pivotal roles as bacterial virulence factors. These molecules are potent pathogen-associated molecular patterns (PAMPs) capable of triggering profound immune responses. At Creative Biolabs, we specialize in dissecting these complex structures. Through our advanced Anti-Bacterial Gram-Negative Glycan (LPS) Antibody Development and Anti-Bacterial Gram-Positive Glycan (LTA) Antibody Development services, we empower researchers to investigate the structural nuances of endotoxins, map their roles in pathogenesis, and develop novel therapeutic or diagnostic tools targeting these critical glycans.
Architecture of the Bacterial Cell Envelope
To understand the function of bacterial endotoxins, one must first appreciate the architectural dichotomy between Gram-negative and Gram-positive bacteria. The fundamental difference lies in the composition and thickness of the peptidoglycan layer and the presence of an outer membrane.
Gram-Negative Bacteria
Gram-negative bacteria possess a thin layer of peptidoglycan sandwiched between an inner cytoplasmic membrane and a unique outer membrane. This outer membrane is asymmetric; its inner leaflet is composed of phospholipids, while its outer leaflet is predominantly composed of lipopolysaccharide (LPS). This LPS barrier provides resistance against hydrophobic antibiotics and detergents, serving as a primary line of defense.
Gram-Positive Bacteria
In contrast, Gram-positive bacteria lack an outer membrane but feature a substantially thicker peptidoglycan layer (20–80 nm). Threaded through this mesh are unique anionic polymers known as Teichoic Acids (TAs). Those covalently anchored to the underlying cell membrane lipids are termed Lipoteichoic Acids (LTA), while those attached to the peptidoglycan are Wall Teichoic Acids (WTA). LTA extends to the cell surface, acting as a major surface antigen and regulator of cell wall enzymes.
Detailed Structural and Chemical Features
The biological activity of bacterial glycans is strictly dictated by their fine chemical structure. Small modifications in acylation patterns or sugar composition can drastically alter immunogenicity and toxicity.
Lipopolysaccharide (LPS) Structure
LPS is the specific endotoxin of Gram-negative bacteria. Its tripartite structure allows it to function simultaneously as a structural anchor, a stability factor, and a highly variable shield against host immunity.
| Domain | Structural Composition | Biological Function |
|---|---|---|
| Lipid A | Phosphorylated glucosamine disaccharide backbone acylated with fatty acid chains (typically 4-7 chains). The degree of acylation (e.g., hexa-acylated in E. coli vs. tetra-acylated in Y. pestis) is critical. | Hydrophobic anchor; the "toxic center" responsible for endotoxic activity via TLR4 activation. Modifications here are key to immune evasion. |
| Core Oligosaccharide |
Inner Core: Kdo (3-deoxy-D-manno-oct-2-ulosonic acid) and heptose residues. Outer Core: Hexose sugars (glucose, galactose, GlcNAc). |
Provides structural integrity and cross-linking via divalent cations (Ca²⁺, Mg²⁺), stabilizing the outer membrane. Highly conserved within genera. |
| O-Antigen | Repeating oligosaccharide units (O-units) extending outwards. Highly variable sugar composition (e.g., tyvelose, abequose). | Determines serological specificity (serotype); protects against complement-mediated lysis and phagocytosis. Can be lost in "rough" mutants. |
Lipoteichoic Acid (LTA) Structure
Lipoteichoic acid serves as the functional equivalent of LPS in Gram-positive organisms (e.g., Staphylococcus aureus, Bacillus subtilis). Structurally, LTA is an amphiphile formed by a glycolipid anchor and a long hydrophilic chain.
| Component | Structural Detail | Functional Role |
|---|---|---|
| Poly-Glycerol Phosphate Backbone | Consists of repeating 1,3-linked glycerol phosphate units. The chain length is variable and species-specific. | Forms the main hydrophilic scaffold extending through the peptidoglycan layer. |
| Functional Substitutions | Hydroxyl groups are substituted with D-alanine esters or glycosyl residues (GlcNAc, GalNAc). | Neutralizes negative charge (D-alanine), regulates autolysins, and modulates susceptibility to cationic peptides. |
| Glycolipid Anchor | Typically a diglucosyl-diacylglycerol (Glc2-DAG) moiety. | Anchors the polymer into the cell membrane; lipid tail length affects membrane stability. |
Biosynthetic Pathways Comparison
Understanding biosynthesis is crucial for identifying drug targets. The pathways for assembling the outer membrane (LPS) and the cell wall (LTA) share common precursors but diverge significantly in their cellular location and transport mechanisms.
LPS Biosynthesis (Gram-Negative):
- Cytoplasmic Assembly: Lipid A and Core are assembled on the inner membrane surface.
- Flipping & Transport: The MsbA transporter flips the core-lipid A to the periplasm.
- Ligation: The WaaL ligase attaches the O-antigen (synthesized separately on undecaprenyl phosphate).
- Export: The Lpt machinery transports the mature LPS to the cell surface.
LTA Biosynthesis (Gram-Positive):
- Extracellular Polymerization: LtaS enzyme polymerizes the glycerol phosphate chain outside the membrane.
- D-Alanylation: D-alanine is added intracellularly and flipped out by the DltABCD system.
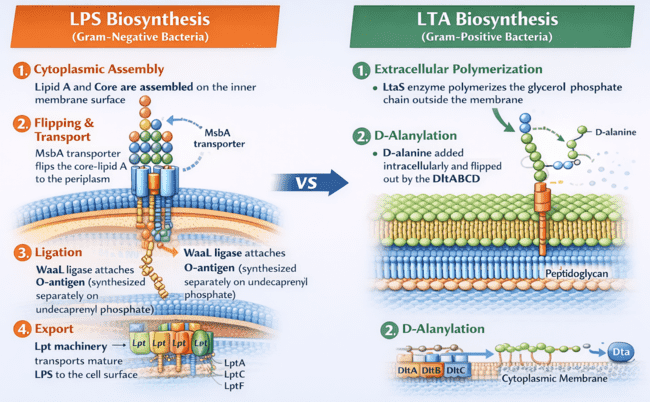
Fig.1 Comparison of LPS vs LTA Biosynthesis Pathways
Mechanisms in Pathogenicity and Immunity
Both LPS and LTA act as potent immunostimulants, but they utilize distinct signaling pathways to alert the host immune system. The dysregulated activation of these pathways leads to the "cytokine storm" characteristic of septic shock.
TLR4 Signaling (LPS)
The endotoxin mechanism for LPS is highly specialized. In the bloodstream, LPS Binding Protein (LBP) extracts LPS aggregates and transfers monomeric LPS to CD14. CD14 then presents LPS to the myeloid differentiation factor 2 (MD-2), which is complexed with Toll-like Receptor 4 (TLR4). The binding of Lipid A to the hydrophobic pocket of MD-2 induces dimerization of the TLR4/MD-2 complex. This recruits intracellular adaptors (MyD88 and TRIF), initiating a cascade that results in the translocation of NF-κB and the release of pro-inflammatory cytokines like TNF-α, IL-1β, and IL-6.
TLR2 Signaling (LTA)
LTA is primarily recognized by Toll-like Receptor 2 (TLR2), which forms heterodimers with TLR1 or TLR6 depending on the ligand's acylation pattern. While the downstream signaling also utilizes the MyD88 pathway to activate NF-κB, the kinetics and intensity of the response often differ from that of LPS. Variations in the glycolipid anchor structure and the degree of D-alanine substitution on the LTA backbone significantly influence its potency as an inflammatory agonist.
Virulence and Immune Evasion
Bacteria have evolved sophisticated mechanisms to modify their surface glycans to evade detection:
- Lipid A Modification: Pathogens like Salmonella and Yersinia can modify their Lipid A (e.g., by palmitoylation or addition of aminoarabinose) to reduce TLR4 recognition and resist antimicrobial peptides.
- Molecular Mimicry: Some bacteria (e.g., Campylobacter jejuni, Neisseria meningitidis) express LOS or capsular polysaccharides that structurally mimic human gangliosides or sialic acid, allowing them to hide from the immune system ("Wolf in sheep's clothing").
- Biofilm Formation: LTA plays a structural role in the formation of biofilms in Staphylococci, protecting the bacteria from antibiotics and immune clearance.
Potential as Therapeutic and Diagnostic Targets
The conserved yet specific nature of bacterial cell wall glycans makes them ideal targets for medical intervention.
Vaccine Design
Glycoconjugate Vaccines: Since polysaccharides are T-cell independent antigens, they induce weak memory. Conjugating them to carrier proteins (e.g., CRM197) recruits T-cell help. We assist in evaluating the immunogenicity of these conjugates.
Antibody Therapy
Neutralizing Antibodies: Monoclonal antibodies targeting conserved Lipid A regions can potentially neutralize endotoxin in sepsis. Opsonic antibodies against O-antigens facilitate phagocytosis of multidrug-resistant bacteria.
Diagnostic Markers
Rapid Detection: The O-antigen specificity allows for precise serotyping (e.g., distinguishing E. coli O157:H7 from commensal strains) using lateral flow assays or agglutination tests.
Research Tools and Technical Needs
Investigating these complex structures requires specialized tools that can differentiate between subtle structural variations (e.g., Lipid A acylation states or O-antigen serotypes). Creative Biolabs provides a comprehensive suite of services designed for bacteriology and immunology research.
Specificity is Key: Our Antibody Services
Researchers often ask: "How do I obtain a monoclonal antibody against a specific LPS serotype?" or "Is there a broad-spectrum antibody for Lipid A?". Creative Biolabs addresses these needs through:
- Anti-LPS Antibody Development: Generation of high-affinity antibodies targeting specific O-antigens (for serotyping), Core regions, or Lipid A moieties (for broad detection).
- Anti-LTA Antibody Development: Production of mAbs against the poly-glycerol phosphate backbone or specific glycosyl substitutions of Gram-positive bacteria.
- Functional Assays: We offer neutralization assays (Limulus Amebocyte Lysate - LAL alternative), opsonophagocytic killing assays (OPKA), and biofilm inhibition studies.
Inquire About Anti-LPS Antibody Services
Featured Services
To support advanced research in bacterial glycobiology, Creative Biolabs offers specialized platforms ranging from high-throughput screening to custom synthesis.
A high-throughput platform featuring diverse bacterial polysaccharides and LPS structures for rapid profiling of antibody specificity and host-pathogen interactions.
View Service »Comprehensive structural elucidation of bacterial glycans using advanced Mass Spectrometry (MS) and NMR, identifying composition, linkage, and modifications.
View Service »Tailored enzymatic or chemical conjugation services to attach specific bacterial glycan antigens to proteins or nanoparticles for vaccine development.
View Service »Published Data
The elucidation of bacterial surface glycosignatures is pivotal for understanding pathogenesis and developing targeted interventions. A seminal 2020 review details the application of high-throughput microarray technologies to decipher the complex landscape of bacterial cell wall glycans. These platforms enable the simultaneous analysis of diverse surface conjugates, including the defining Lipopolysaccharides (LPS) of Gram-negative envelopes and the Lipoteichoic Acids (LTA) characteristic of Gram-positive architectures. By immobilizing broad libraries of natural and synthetic glycan probes, researchers can rapidly map the specific interactions between these virulence factors and host glycan-binding proteins, such as C-type lectins and antibodies. The literature underscores the utility of these arrays in identifying conserved epitopes across serotypes, a critical step for vaccine design. Furthermore, the analysis reveals how subtle structural variations in cell wall components—such as O-antigen diversity or unique mycobacterial glycolipids—dictate immune recognition and evasion strategies. This molecular-level profiling not only aids in serodiagnosis but also accelerates the discovery of high-affinity ligands for therapeutic development, validating the cell wall as a rich reservoir of druggable targets.
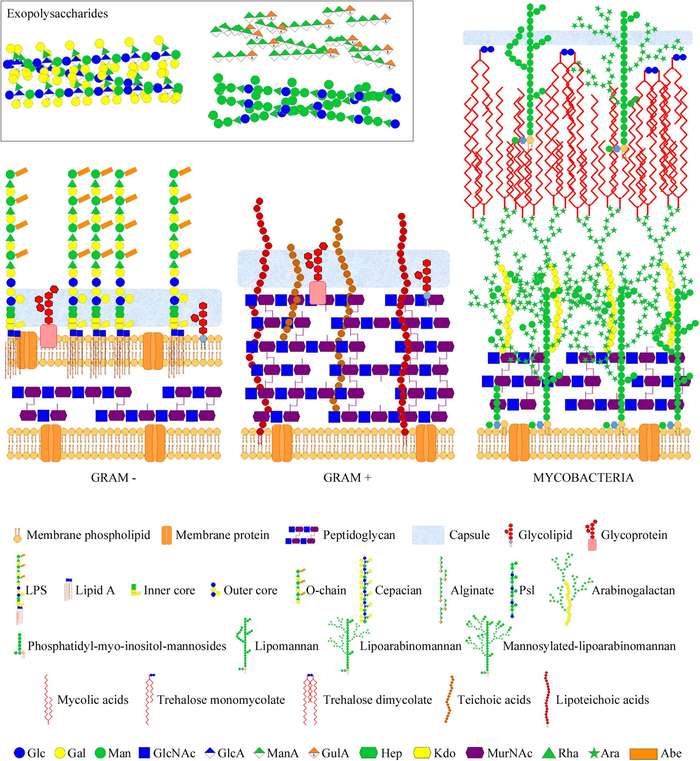 Fig.2
Structural diversity of bacterial cell envelopes showing the differential arrangement of key glycan virulence factors including LPS and LTA.1
Fig.2
Structural diversity of bacterial cell envelopes showing the differential arrangement of key glycan virulence factors including LPS and LTA.1
FAQs
What is the difference between LPS and LOS?
Lipopolysaccharides (LPS) contain three regions: Lipid A, the Core oligosaccharide, and the O-antigen polysaccharide. Lipooligosaccharides (LOS), found in bacteria like Neisseria and Haemophilus, lack the O-antigen region, consisting only of Lipid A and an extended core oligosaccharide. LOS is chemically analogous to the "rough" LPS mutants of enterobacteria.
Why is Lipid A considered the "endotoxic center" of LPS?
Lipid A is the highly conserved, hydrophobic anchor of LPS. Its specific structure—typically a bis-phosphorylated glucosamine disaccharide with 6 acyl chains (in E. coli)—is the specific ligand recognized by the TLR4/MD-2 complex. Variations in the number of acyl chains (e.g., tetra-acylated Lipid A) can render the molecule antagonistic or non-stimulatory to human TLR4.
Can antibodies distinguish between different LPS serotypes?
Yes. The O-antigen region of LPS is highly variable between strains of the same species (determining the serotype). We can develop monoclonal antibodies that specifically recognize these unique O-antigen repeat units for serotyping, as well as antibodies targeting the conserved Core or Lipid A regions for broad-spectrum detection.
How can I evaluate the efficacy of a glycoconjugate vaccine candidate?
To evaluate a vaccine, you need to assess the functional activity of the induced antibodies. We recommend using Opsonophagocytic Killing Assays (OPKA) to measure the antibodies' ability to promote bacterial killing by phagocytes, as well as serum bactericidal assays (SBA) for complement-mediated killing.
Can you develop an ELISA kit for detecting specific LTA structures?
Yes, we can develop sandwich or competitive ELISA kits tailored to your specific LTA target. By generating high-affinity antibodies against the unique glycolipid anchor or the D-alanylated backbone, we can create sensitive diagnostic tools for Gram-positive bacterial infections.
Reference:
- Campanero-Rhodes, M. A., Palma, A. S., Menéndez, M., and Solís, D. "Microarray Strategies for Exploring Bacterial Surface Glycans and Their Interactions With Glycan-Binding Proteins." Frontiers in Microbiology 10 (2020): 2909. Distributed under the terms of the CC BY 4.0. https://doi.org/10.3389/fmicb.2019.02909
