Anti-Bacterial (Gram-Negative) Glycan & LPS Antibody Development Service
The cell envelope of Gram-negative bacteria is a complex, multi-layered structure dominated by unique glycans and lipopolysaccharides (LPS). These surface molecules are critical for bacterial structural integrity, protection against host defenses, and virulence. Consequently, they serve as primary targets for diagnostic assays, serotyping, and therapeutic interventions. Creative Biolabs leverages decades of expertise in glyco-immunology to offer a comprehensive anti-bacterial glycan and LPS antibody development service. Our platform is optimized to overcome the low immunogenicity of carbohydrate antigens, delivering high-affinity antibodies that can discriminate between specific serotypes or recognize broad-spectrum core structures.
Background: Structure and Targets on the Gram-Negative Surface
The development of an effective gram negative bacteria antibody requires a deep understanding of the diverse glycan structures present on the bacterial surface. The most prominent of these is Lipopolysaccharide (LPS), also known as endotoxin, which covers nearly 75% of the outer membrane surface. Additionally, many pathogenic strains are encapsulated by capsular polysaccharides (K-antigens) that mask underlying structures and inhibit phagocytosis. Understanding the nuanced differences between smooth-type (possessing full O-antigen) and rough-type (lacking O-antigen) strains is vital for selecting the correct target for your research application.
Lipopolysaccharide (LPS) Components
LPS is a tripartite molecule, and antibodies can be engineered to target each distinct region for different research applications.
Lipid A
Core Oligosaccharide
O-Antigen (O-Polysaccharide)
Capsular Polysaccharides (K-Antigens)
Many invasive bacteria, such as Neisseria meningitidis, Klebsiella pneumoniae, and certain E. coli strains, possess a polysaccharide capsule. We develop K-antigen antibody tools and neisseria meningitidis capsule antibody reagents to aid in the study of vaccine efficacy and bacterial evasion mechanisms. The capsule often acts as a cloak, preventing the binding of antibodies to the underlying LPS, making anti-capsular antibodies critical for studying virulence in encapsulated strains.
Challenges in Developing Antibodies Against Bacterial Glycans
Low Immunogenicity
Bacterial glycans, particularly pure polysaccharides, are T-cell independent antigens. They typically fail to induce a germinal center reaction, resulting in low-affinity IgM antibodies without memory B-cell formation.
Structural Heterogeneity
LPS is not a single molecule but a population of varying chain lengths. This heterogeneity makes it difficult to generate a monoclonal antibody that recognizes a consistent epitope across different bacterial preparations.
Aggregation & Solubility
The amphipathic nature of LPS, primarily due to the hydrophobic Lipid A, causes it to form micelles or aggregates in aqueous solution. This can mask epitopes, particularly those in the core region, preventing antibody binding.
Cross-Reactivity Risks
Shared core structures or similar monosaccharide compositions between species can lead to unwanted cross-reactivity. Developing a truly strain-specific antibody requires rigorous counter-screening against closely related pathogens.
Our Solutions: Custom Anti-Lipopolysaccharide (LPS) & Glycan Antibody Services
To address the challenges mentioned above, we have developed a suite of specialized services. Our service portfolio covers the entire spectrum of the gram negative cell wall antibody field. We can tailor the specificity of your antibody to meet precise research goals.
Strain-Specific Anti-LPS Antibody Development
Focusing on the variable O-antigen region, we develop antibodies capable of distinguishing between closely related serotypes. This service is vital for food safety testing and clinical diagnostics where precise identification of the pathogen strain is required. We utilize whole-bacteria immunization strategies combined with precise boosting to elicit high-titer IgG responses against specific O-antigens.
Broad-Spectrum Anti-Core & Lipid A Antibody
By targeting the conserved Inner Core (containing KDO) or Lipid A, we generate endotoxin antibody reagents that recognize a wide range of Gram-negative bacteria. These tools are indispensable for measuring total endotoxin load, developing pan-Gram-negative detection assays, and studying the conserved mechanisms of septic shock. We employ specialized detoxification protocols to use Lipid A as an immunogen without causing toxicity in the host animal.
Anti-Capsular Polysaccharide (K-Antigen) Antibody
We provide specialized development for antibodies against the thick polysaccharide capsules of bacteria like Klebsiella and Neisseria. These antibodies are crucial for vaccine potency testing and for investigating how bacteria evade complement-mediated killing. Our team uses specific conjugation chemistries to link these polysaccharides to carrier proteins, converting them into T-cell dependent antigens.
Functional & Neutralizing Antibody Development
Beyond simple binding, we screen for anti-LPS neutralizing antibody candidates that can block the biological activity of endotoxins or mediate bactericidal activity. These functional antibodies are essential tools for preclinical research into sepsis therapeutics and passive immunization strategies. We validate these candidates using in vitro LAL assays and cell-based cytokine release assays (e.g., TNF-alpha inhibition).
Workflow for Anti-LPS and Gram-Negative Bacteria Antibody Generation
Request a Quote for LPS Antibody Development
Key Advantages of Our Gram-Negative Antibody Development Platform
High Specificity
Optimized screening protocols designed to eliminate cross-reactivity and ensure precise binding to the target glycan moiety, distinguishing between serotypes.
Diverse Host Options
Full availability of Mouse, Rabbit, and Llama (VHH) platforms to generate different antibody formats (IgG, IgM, scFv, Fab) suitable for various diagnostic and therapeutic applications.
Endotoxin-Free
Rigorous purification processes to provide antibody products with ultra-low endotoxin levels, making them suitable for sensitive cell-based and in vivo assays.
Tailored Validation
Validation on specific bacterial strains (e.g., Pseudomonas aeruginosa, Salmonella) relevant to your research, using flow cytometry and bactericidal assays.
Published Data
Recent studies have highlighted the therapeutic potential of anti-LPS monoclonal antibodies in combating multi-drug resistant infections. In a 2023 study, researchers developed high-affinity IgG2b monoclonal antibodies, designated WVDC-0357 and WVDC-0496, targeting the O-antigen lipopolysaccharide of Pseudomonas aeruginosa. These antibodies demonstrated significant functional activity in vitro, directly reducing bacterial viability and mediating strong bacterial agglutination.
The protective efficacy of these pseudomonas aeruginosa LPS antibody candidates was evaluated in a lethal murine sepsis model. Prophylactic administration of the antibodies at doses as low as 15 mg/kg conferred 100% survival against a lethal bacterial challenge, whereas control mice succumbed to the infection (Fig.1). Furthermore, in acute pneumonia models, treatment significantly reduced bacterial burden in the lungs and attenuated the production of inflammatory cytokines. This data underscores the value of targeting the O-antigen for both therapeutic and vaccine development strategies.
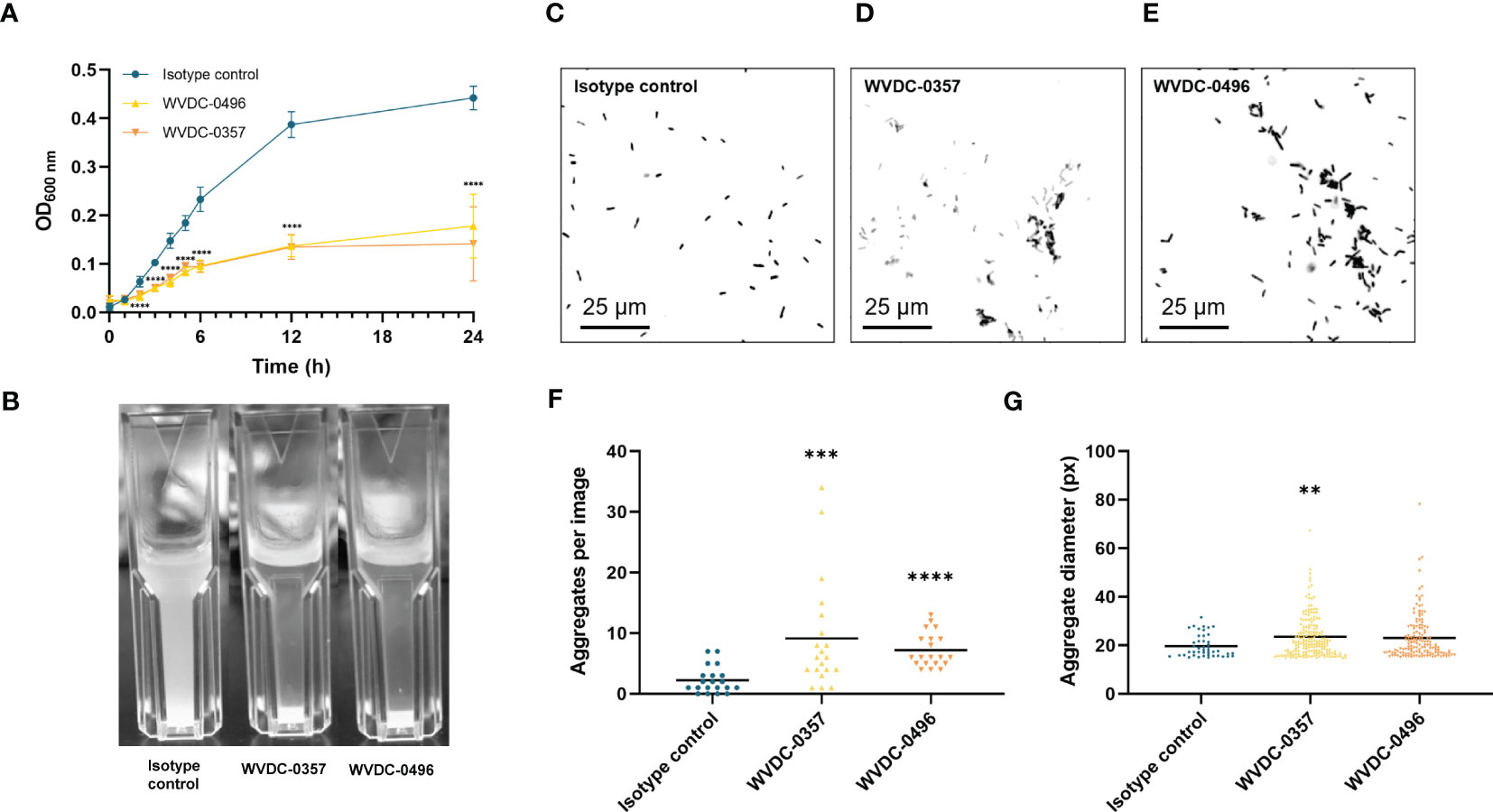 Fig.1 Protective efficacy of anti-LPS monoclonal antibodies in a lethal P. aeruginosa sepsis model.1
Fig.1 Protective efficacy of anti-LPS monoclonal antibodies in a lethal P. aeruginosa sepsis model.1
FAQs
What is the difference between Anti-Lipid A and Anti-O-Antigen antibodies?
An anti-lipid A antibody targets the conserved hydrophobic region of LPS. It typically exhibits broad cross-reactivity across many Gram-negative species and is useful for detecting total endotoxin. In contrast, an anti-O-antigen antibody binds to the outermost polysaccharide chain, which is highly variable. These antibodies are strain-specific and are used for serotyping (e.g., distinguishing E. coli O157 from O111).
Can you develop antibodies for LPS detection in complex samples?
Yes. We can develop high-sensitivity LPS detection antibody pairs suitable for Sandwich ELISA. These are optimized to detect free LPS or whole bacteria in complex matrices like serum, food homogenates, or environmental water samples.
Do you offer antibodies against the Core Oligosaccharide region?
Yes. We develop anti-LPS core antibody reagents, including those targeting the KDO region (KDO antibody). Since the core structure is more conserved than the O-antigen but more accessible than Lipid A, these antibodies are excellent tools for broad-spectrum detection of Gram-negative bacteria.
What immunogens do you use for antibody generation?
Depending on the project goal, we use purified LPS, delipidated polysaccharides conjugated to carrier proteins (like KLH), or heat-inactivated whole bacteria. This flexibility ensures we generate antibodies with the optimal binding profile for your specific application.
Reference:
- Hornback, Michael, et al. "Monoclonal antibodies against lipopolysaccharide protect against Pseudomonas aeruginosa challenge in mice." Frontiers in Cellular and Infection Microbiology 13 (2023): 1191806. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3389/fcimb.2023.1191806
