Anti-GAG Sulfation Motif (Neo-epitope) Antibody Development Service
Glycosaminoglycans (GAGs) are sophisticated linear polysaccharides that orchestrate critical biological interactions at the cell surface and within the extracellular matrix (ECM). Their functional diversity is not encoded by a simple genetic template but is instead generated through complex post-translational modifications, primarily specific patterns of sulfation. This "sulfation code" dictates how GAGs interact with a vast array of binding partners, including growth factors, chemokines, and viral envelope proteins. At Creative Biolabs, we provide a highly specialized GAG antibody development platform dedicated to the development of antibodies that target these precise anti-GAG sulfation motifs. Our approach moves beyond generic carbohydrate recognition to generate high-affinity reagents capable of distinguishing subtle structural isomers and neo-epitopes that emerge during pathological conditions such as cancer metastasis and tissue fibrosis.
Decoding the Sulfation Motifs: A Target for Precision Medicine
The structural complexity of GAGs, such as heparan sulfate (HS) and chondroitin sulfate (CS), arises from the combinatorial modification of their disaccharide building blocks. While the "backbone" of these chains is often conserved, the specific placement of sulfate groups creates unique binding interfaces. In healthy tissue, these modifications are tightly regulated. However, in disease states, the expression of sulfotransferases and sulfatases is often dysregulated, leading to the presentation of aberrant, tumor-associated GAG structures. These neo-epitopes are virtually absent in normal adult tissues, making them ideal targets for therapeutic intervention and diagnostic imaging.
Developing antibodies against these targets is notoriously difficult due to the low immunogenicity of carbohydrates and the high degree of structural similarity between different sulfation patterns. For example, distinguishing a 4,6-disulfated CS-E unit from a mixture of 4-sulfated CS-A and 6-sulfated CS-C requires an antibody with exquisite conformational specificity. Similarly, targeting the rare 3-O-sulfation in heparan sulfate, which is crucial for viral entry and anticoagulant activity, demands a strategy that can bypass the immune system's tolerance to self-antigens. Our service is designed to overcome these barriers, providing researchers with tools to dissect the functional roles of specific glycan motifs in oncology, neuroscience, and infectious disease.
Advanced Custom GAG Motif Antibody Services
We do not simply offer generic anti-GAG antibody production. Our platform is engineered to address the most significant bottleneck in glycan research: specificity. By combining synthetic carbohydrate chemistry with advanced immune engineering, we deliver antibodies that target defined micro-structures within the GAG chain.
Cryptic Neo-Epitope Discovery & Targeting
Many high-value therapeutic targets are "cryptic," meaning they are either masked by protein interactions or present in low abundance within the native GAG chain. We specialize in generating antibodies against these elusive targets, such as the CS-E motif (GlcA-GalNAc4S,6S), which acts as a major receptor for VEGF and facilitates tumor metastasis in ovarian and breast cancers. We also target the HS-NS6S motif, a specific heparan sulfate domain associated with glioblastoma invasiveness. By using chemically synthesized, multivalent neo-glycoconjugates as immunogens, we can focus the immune response specifically on these rare motifs, avoiding the generation of antibodies against the abundant, non-specific GAG backbone.
Isomer-Specific Antibody Engineering
A critical challenge in GAG biology is distinguishing between structural isomers that have the same molecular weight but different biological functions. Standard immunization often results in cross-reactive polyclonal mixtures. Our service utilizes a rigorous subtractive screening protocol to isolate clones that can discriminate between closely related structures, such as distinguishing CS-A (4-sulfated) from CS-E (4,6-disulfated), or HS-2S (2-O-sulfated) from HS-6S (6-O-sulfated). We ensure that the final monoclonal antibody exhibits high selectivity (>100-fold difference in binding affinity), providing a reliable tool for precise structural mapping and functional inhibition assays.
Technical Workflow for Sulfation-Specific Antibody Generation
Request a Proposal
Why Choose Our GAG-Specific Antibodies?
Defined Specificity
Validated against large panels of GAG isomers to ensure no cross-reactivity (e.g., anti-CS-E will not bind CS-A).
Neo-epitope Focus
Strategies optimized for isolating antibodies against tumor-specific glycosaminoglycan antibody targets.
Versatile Formats
Available as IgG, IgM, or recombinant scFv formats, tailored for IHC, ELISA, or therapeutic development.
Cost-Effective
Efficient screening pipelines reduce the time and cost associated with difficult glycan targets.
How to Start Your Project
Initiating a custom anti-GAG antibody project is straightforward. Contact our team with your target of interest. We will consult with you to determine the best immunogen strategy—whether it involves using a defined neoepitope GAG antibody approach or a broader library screen. Once the scope is defined, we provide a detailed project roadmap, from antigen synthesis to final clone delivery.
Consult an Expert
Published Data
Glycosaminoglycans (GAGs) undergo extensive structural remodeling during malignant transformation, creating tumor-specific "sulfation codes." A study illustrates the critical importance of targeting these specific motifs. The researchers utilized the single-chain variable fragment (scFv) antibody GD3G7, selected via phage display to specifically recognize the 4,6-disulfated chondroitin sulfate E (CS-E) motif.
In the Figure 1, the specificity of this antibody is rigorously validated using immunohistochemistry on primary serous ovarian carcinoma tissues. While generic antibodies against total chondroitin sulfate (clone IO3H10) and dermatan sulfate (clone GD3A12) showed broad, non-specific staining across the tissue, the anti-CS-E antibody GD3G7 revealed a highly distinct pattern. It specifically stained the intratumoral stroma and tumor-associated capillaries, with negligible binding to healthy regions. This differential staining highlights the antibody's ability to discriminate the tumor-associated CS-E neo-epitope from the background of normal stromal GAGs. Such high-precision tools are indispensable for dissecting the roles of GAG sulfation in metastasis and for developing targeted therapies that spare healthy tissues.
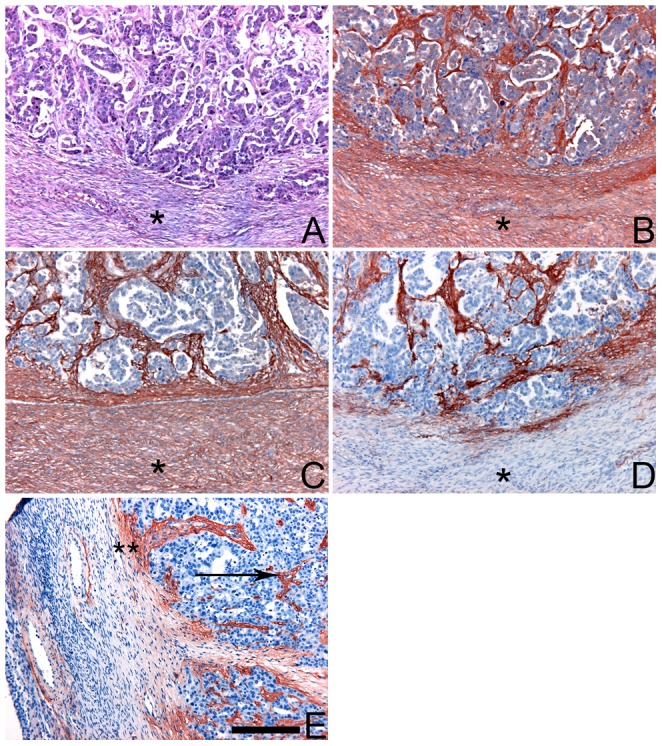 Fig.1 Anti-CS-E antibody (GD3G7) reveals tumor-selective GAG neo-epitope.1
Fig.1 Anti-CS-E antibody (GD3G7) reveals tumor-selective GAG neo-epitope.1
FAQs
How do you ensure the antibody doesn't cross-react with other sulfated GAGs?
We utilize a comprehensive counter-screening strategy. During the selection phase, clones are incubated not only with the target antigen (e.g., HS-NS6S) but also with high concentrations of structurally related GAGs (e.g., unmodified HS, CS, DS). Only clones that bind the target and fail to bind the competitors are selected for further development.
Can you develop antibodies against very short GAG sequences (neo-epitopes)?
Yes. This is a core strength of our neo-epitope GAG antibody development service. We can synthesize defined oligosaccharides (as short as disaccharides or tetrasaccharides) containing the specific sulfation motif of interest and conjugate them to carrier proteins to focus the immune response on the exact neo-epitope.
What applications are these antibodies suitable for?
Our custom GAG antibodies are typically validated for ELISA and Dot Blot. Depending on the project scope, we can also perform validation for Immunohistochemistry (IHC) on tissue microarrays and Flow Cytometry on specific cell lines to ensure they work in your intended assay.
Do you offer recombinant production of these antibodies?
Yes. Once a hybridoma or phage clone is identified, we can sequence the variable regions (VH/VL) and clone them into expression vectors. This allows for the production of recombinant anti-heparan sulfate antibodies or other GAG antibodies with consistent batch-to-batch performance and the ability to engineer Fc regions.
Reference:
- Vallen, M. J., et al. "Primary ovarian carcinomas and abdominal metastasis contain 4, 6-disulfated chondroitin sulfate rich regions, which provide adhesive properties to tumour cells." PLoS One 9.11 (2014): e111806. Distributed under Open Access license CC BY 4.0. https://doi.org/10.1371/journal.pone.0111806
Supports
- Anti-Heparan Sulfate (HS) Antibody Development Service
- Anti-Chondroitin Sulfate (CS) Antibody Development Service
- Anti-Dermatan Sulfate (DS) Antibody Development Service
- Anti-Keratan Sulfate (KS) Antibody Development Service
- Anti-Hyaluronic Acid (HA) Antibody Development Service
- Anti-GAG Sulfation Motif (Neo-epitope) Antibody Development Service
- Tumor-Associated GAG Antibody Development Service
