Custom Glycopeptide Target Antibody Development Service
The landscape of cancer immunotherapy is shifting towards targets with higher tumor specificity and lower on-target, off-tumor toxicity. Glycopeptides, representing the unique structural interface between protein backbones and post-translational glycosylation, are among the most promising neo-antigens in modern oncology. In healthy tissues, heavy glycosylation often masks the protein core of mucins and other cell surface glycoproteins. However, in cancer, the deregulation of glycosyltransferases leads to aberrant glycosylation, exposing novel epitopes—such as truncated O-glycans (Tn, sTn) on mucins or sialylated structures on cell surface proteins—that are virtually absent in healthy somatic tissues.
At Creative Biolabs, we specialize in custom glycopeptide antibody development. Our advanced platform overcomes the notorious non-immunogenicity of carbohydrates by utilizing precision-synthesized glycopeptide immunogens and advanced library screening strategies. We deliver high-affinity reagents capable of distinguishing tumor-specific glyco-forms, such as the MUC1-Tn antibody or anti-MUC16 monoclonal antibody, empowering your research into cancer diagnostics, CAR-T cell therapy, and Antibody-Drug Conjugate (ADC) development. Our goal is to provide researchers with the precise tools needed to unlock the therapeutic potential of the glycoproteome.
The Challenge: Why Glycopeptide Antibodies are Difficult to Generate
Immunological Tolerance
The protein backbones of target antigens like MUC1 or CD43 are self-antigens. The immune system of standard host animals often possesses strong tolerance mechanisms that prevent the generation of high-affinity antibodies against these self-proteins, even when they are aberrantly glycosylated. Breaking this tolerance requires specialized immunization strategies.
Weak Immunogenicity of Glycans
Carbohydrate antigens are generally T-cell independent antigens that elicit weak immune responses, primarily IgM with low affinity. Generating class-switched, high-affinity IgG antibodies that specifically recognize the small glycan epitope in the context of a peptide sequence is a significant hurdle in hybridoma technology.
Structural Heterogeneity
Natural glycoproteins are heterogeneous mixtures of glycoforms. Using native proteins as immunogens often leads to a polyclonal response directed against dominant protein epitopes rather than the specific, tumor-associated glycopeptide interface. This necessitates the use of chemically defined synthetic immunogens to focus the immune response.
Workflow: From Design to Deliverable
Our comprehensive workflow is designed to address the specific challenges of glycopeptide immunogenicity. By combining chemical precision with biological diversity, we ensure the generation of antibodies that are strictly specific to the combined glycan-peptide epitope.
Request Your Glycopeptide Antibody Project
Specific Glycopeptide Target Services
We offer a comprehensive suite of development services covering the most clinically relevant mucins and cell surface glycoproteins. Whether you need an anti-MUC1 monoclonal antibody for breast cancer research or a gastric cancer MUC5AC antibody for diagnostic assay development, our team has the expertise to deliver.
MUC1 Glycopeptide Antibodies
MUC1 is the gold standard for glycopeptide targets. In epithelial cancers, the extracellular Variable Number Tandem Repeat (VNTR) region becomes underglycosylated, exposing the peptide backbone adorned with truncated sugars like Tn and sTn. This creates highly specific tumor neo-epitopes. We develop reagents including:
- MUC1-Tn antibody: Targets the VNTR peptide modified with GalNAc (Tn antigen), a hallmark of breast and pancreatic cancer.
- MUC1-STn antibody: Specific for the sialylated Tn structure on MUC1, associated with poor prognosis and metastasis.
- MUC1 VNTR antibody: Recognizing the peptide backbone exposed due to underglycosylated MUC1 antibody profiles.
- MUC1-N antibody: Targeting the large N-terminal subunit released into the circulation, ideal for serum biomarker assays.
MUC16 (CA125) & MUC4 Antibodies
MUC16 (CA125) is a massive mucin overexpressed in ovarian cancer, while MUC4 is a key marker in pancreatic malignancy. Aberrant glycosylation on these large proteins creates unique therapeutic windows. Our service provides:
- Anti-MUC16 monoclonal antibody: High-affinity binders for the glycosylated MUC16 antibody epitope, distinguishing tumor-derived CA125 from mesothelial forms.
- Pancreatic cancer MUC4 antibody: Specific for the sialylated MUC4 antibody forms found in pancreatic ductal adenocarcinoma (PDAC).
Secreted Mucin Antibodies (MUC2, MUC5AC)
Gel-forming mucins like MUC2 and MUC5AC are critical markers in gastrointestinal cancers. Changes in their glycosylation status serve as early diagnostic indicators for colorectal and gastric malignancies. We generate:
- Colon cancer MUC2 antibody: Detecting aberrant glycosylation on the intestinal mucin antibody target MUC2, often identifying "immature" glycosylation in tumors.
- Gastric cancer MUC5AC antibody: Specific for the tumor-associated forms of this secreted mucin antibody, useful for distinguishing cancer subtypes.
CD43 & PODXL Glycopeptide Antibodies
Beyond mucins, large transmembrane proteins like Leukosialin (CD43) and Podocalyxin (PODXL) exhibit cancer-specific glycosylation changes. CD43, typically restricted to leukocytes, is aberrantly expressed in solid tumors with altered glycosylation. Our development pipeline includes:
- Anti-CD43 antibody: Targeting the tumor associated CD43 antibody epitope (often referred to as the UN1 antigen equivalent) found in carcinomas.
- Anti-podocalyxin antibody: Focused on the PODXL glycopeptide antibody interface, serving as a marker for aggressive tumor phenotype.
Highlights of Our Platform
Extreme Specificity
Our antibodies distinguish the glycopeptide from both the naked peptide and the free glycan, ensuring glycosylation site specific antibody binding with minimal off-target effects.
Synthetic Precision
We use homogeneous synthetic glycopeptides as immunogens, eliminating the batch-to-batch variability and heterogeneity associated with biological extracts.
Diverse Hosts
Available in mouse (hybridoma), rabbit (polyclonal/monoclonal), and llama (VHH) formats for anti-glycopeptide antibody generation, suitable for diverse downstream applications.
Versatile Applications
Ideal for IHC, Flow Cytometry, ELISA, and advanced therapeutic engineering like Chimeric Antigen Receptor (CAR) T cells and Antibody-Drug Conjugates (ADCs).
Applications in Cancer Research
CAR-T Cell Therapy
Chimeric Antigen Receptor (CAR) T cells engineered with scFv fragments from a tumor specific MUC1 antibody (e.g., anti-MUC1-Tn) have shown remarkable efficacy in solid tumor models. Unlike standard CARs that target healthy tissues, these glycopeptide-specific CARs selectively kill tumor cells while sparing normal epithelial cells that lack the aberrant glyco-form, thereby minimizing off-tumor toxicity and enhancing the safety profile of the therapy.
Diagnostic Biomarkers
Circulating glycopeptides from secreted mucin antibody targets like MUC5AC or shed fragments of MUC16 (ovarian cancer marker antibody) can be detected in serum. Our custom development service can produce matched antibody pairs that facilitate the development of sensitive sandwich ELISAs. These assays are crucial for early cancer detection, monitoring disease progression, and evaluating patient response to therapy in a non-invasive manner.
Antibody-Drug Conjugates (ADCs)
The rapid internalization rates of certain glycopeptide targets, such as MUC1 and CD43, make them ideal candidates for ADC development. A custom glycopeptide antibody development project at Creative Biolabs can focus on identifying internalizing clones that can effectively deliver cytotoxic payloads directly into the tumor cytoplasm, bypassing the cell membrane barrier and inducing targeted cell death.
Vaccine Efficacy Monitoring
For clinical trials involving glycopeptide-based cancer vaccines (e.g., synthetic MUC1-Tn vaccines), robust tools are needed to measure immunogenicity. Our reagents serve as critical positive controls and capture agents to monitor the patient's immune response, specifically detecting the generation of endogenous anti-glycopeptide antibody titers during the course of vaccination.
Published Data
A recent study published in 2024 illustrates the efficacy of using structure-guided synthetic glycopeptides to generate tumor-specific antibodies. To overcome the weak immunogenicity of self-antigens, researchers designed a vaccine candidate (AuNP-1'-Hnv*) featuring a MUC1 glycopeptide mimetic attached to gold nanoparticles. This immunogen was designed to lock the peptide backbone into the specific bioactive conformation found on tumor cells.
The specificity of the elicited antibodies was validated via confocal microscopy (Fig.1). The imaging results demonstrate that antibodies from immunized mice effectively recognize and bind to native MUC1 expressed on the surface of T47D human breast cancer cells (green fluorescence). Crucially, no staining was observed on MUC1-negative HEK293T cells, confirming that the antibodies are highly specific for the tumor-associated glycopeptide epitope and do not cross-react with non-target cells. This validates our platform's ability to translate synthetic antigen design into biologically functional reagents.
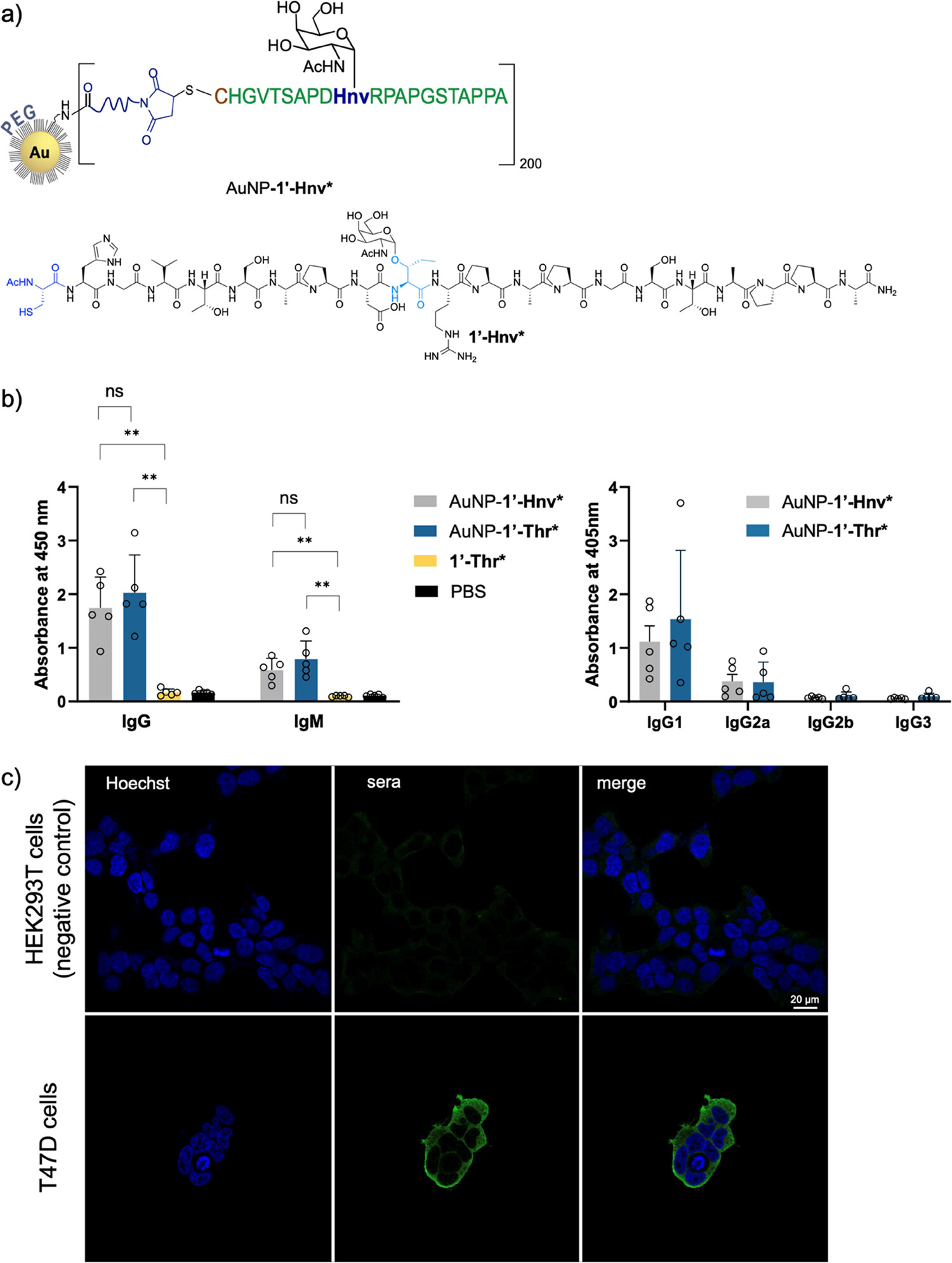 Fig.1 Confocal microscopy analysis of anti-MUC1 antibody specificity.1
Fig.1 Confocal microscopy analysis of anti-MUC1 antibody specificity.1
FAQs
How do you ensure the antibody doesn't bind to the normal protein?
We perform rigorous counter-screening (depletion) steps. During the screening of hybridoma or phage libraries, we incubate the candidates with the non-glycosylated ("naked") peptide and irrelevant glycopeptides. Only clones that bind to the target glycopeptide but fail to bind the naked peptide are selected. This ensures the resulting anti-glycopeptide antibody requires the specific glycan for recognition.
Can you develop antibodies against O-linked vs N-linked glycopeptides?
Yes. We can design immunogens for both. While O-linked glycopeptide antibody projects (targeting Ser/Thr attachment sites like in MUC1) are most common in mucin research, we also have extensive experience with N-linked glycopeptide antibody development for targets where N-glycans (Asn-linked) are the primary tumor-specific modification.
What is the difference between a MUC1-N and MUC1-C antibody?
MUC1 is cleaved into two subunits. MUC1-N is the large extracellular domain containing the VNTRs, which is often shed. MUC1-C is the transmembrane subunit involved in signaling. We can develop a MUC1-N antibody for diagnostic shedding assays or a MUC1-C antibody for therapeutic targeting of the cell-associated oncogenic subunit.
Do you offer validation on tumor tissue microarrays (TMA)?
Yes. We can validate the anti-MUC1 monoclonal antibody or other candidates on TMAs containing both normal and malignant tissues (e.g., breast, pancreas, ovarian, colon). This confirms the aberrant glycosylation antibody profile in a clinically relevant context.
What host species are available for glycopeptide antibody production?
We offer a versatile range of host species to suit different research needs. For monoclonal antibodies, we primarily use mice (hybridoma technology) and rabbits (rabbit monoclonal technology), which are excellent for generating high-affinity binders against difficult epitopes. For polyclonal antibodies, rabbits are the standard. Additionally, we offer llama VHH development for applications requiring small, stable antibody fragments with deep tissue penetration capabilities.
Can you synthesize the glycopeptide immunogen if I only have the protein sequence?
Absolutely. Our chemistry team specializes in glycopeptide synthesis. You only need to provide the protein sequence and the desired glycan modification (e.g., Tn, T, sTn, Sialyl-Lewis X). We will design the optimal immunogen, determining the best conjugation strategy and linker chemistry to present the epitope effectively to the immune system. We handle the entire process from antigen design to antibody delivery.
Reference:
- Pettà, Tommaso, et al. "Structure-Guided Approach for the Development of MUC1-Glycopeptide-Based Cancer Vaccines with Predictable Responses." JACS Au 4.1 (2024): 150-163. Distributed under Open Access license CC BY 4.0. https://doi.org/10.1021/jacsau.3c00587
Supports
- Anti-MUC1 Glycopeptide Antibody Development
- Anti-MUC4 Glycopeptide Antibody Development
- Anti-MUC16 (CA-125) Glycopeptide Antibody Development
- Anti-MUC5AC Glycopeptide Antibody Development
- Anti-MUC2 Glycopeptide Antibody Development
- Anti-Podocalyxin (PODXL) Glycopeptide Antibody Development
- Anti-CD43 Glycopeptide Antibody Development
- Custom Glycopeptide Target Antibody Development
