Glycosaminoglycans (GAGs): Structure, Function, and Pathology
Glycosaminoglycans (GAGs) are long, unbranched polysaccharides consisting of repeating disaccharide units that play a fundamental role in biology. These complex molecules are abundant on cell surfaces and within the extracellular matrix (ECM), where they orchestrate critical physiological processes ranging from cell signaling and hydration to tissue structural integrity. Due to their high polarity and water-attracting properties, GAGs function as essential lubricants and shock absorbers in the body. At Creative Biolabs, we understand the complexity of GAG biology and offer a comprehensive suite of research tools and services to decode their roles in health and disease. For researchers investigating specific GAG interactions, we recommend exploring our Anti-Glycosaminoglycan (GAG) Antibody Development service to accelerate your discovery.
Structure and Classification of Glycosaminoglycans
The fundamental glycosaminoglycans structure involves a repeating disaccharide unit comprising an amino sugar (either N-acetylglucosamine or N-acetylgalactosamine) and a uronic acid (glucuronic acid or iduronic acid) or galactose. GAGs are highly heterogeneous in terms of molecular mass, disaccharide composition, and sulfation patterns. This structural diversity allows them to interact with a vast array of proteins, cytokines, and growth factors.
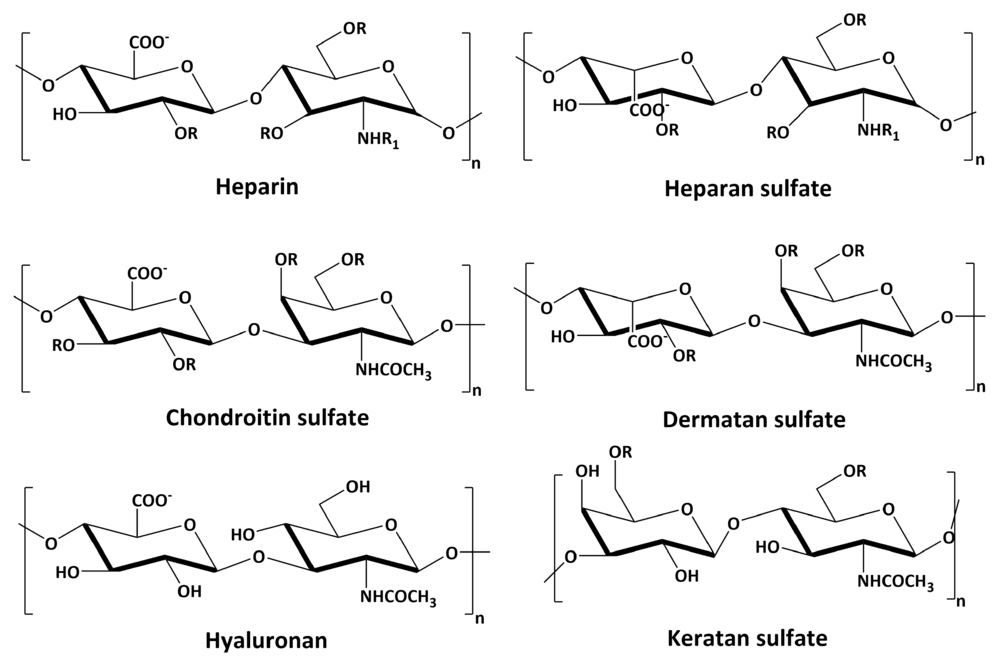 Fig.1 Chemical structures of the repeating disaccharide units of major glycosaminoglycans.1
Fig.1 Chemical structures of the repeating disaccharide units of major glycosaminoglycans.1
Based on their core disaccharide composition, linkage type, and sulfation patterns, GAGs are categorized into four main groups: Hyaluronic Acid (HA), Chondroitin Sulfate/Dermatan Sulfate (CS/DS), Heparan Sulfate/Heparin (HS/Hep), and Keratan Sulfate (KS). With the exception of HA, all GAGs are covalently attached to core proteins to form proteoglycans.
| GAG Type | Disaccharide Unit | Sulfation | Primary Localization & Function |
|---|---|---|---|
| Hyaluronic Acid (HA) | GlcNAc + GlcUA | Non-sulfated | Synovial fluid, vitreous humor, loose connective tissue; facilitates cell migration and tissue hydration. |
| Chondroitin Sulfate (CS) | GalNAc + GlcUA | C4 or C6 sulfated | Cartilage, bone, heart valves; provides resistance to compression and structural support. |
| Dermatan Sulfate (DS) | GalNAc + IdoUA | Variable | Skin, blood vessels, heart valves; involved in wound repair and coagulation regulation. |
| Heparan Sulfate (HS) | GlcNAc + GlcUA/IdoUA | Highly variable | Cell surfaces (syndecans, glypicans), basement membranes; regulates growth factor signaling (FGF, VEGF). |
| Heparin | GlcNAc + IdoUA | Highly sulfated | Mast cell granules; potent anticoagulant activity via Antithrombin III interaction. |
| Keratan Sulfate (KS) | GlcNAc + Gal | C6 sulfated | Cornea, cartilage, bone; maintains corneal transparency and tissue hydration. |
Structural Nuances
Hyaluronic Acid: Unique among GAGs, HA is non-sulfated and synthesized at the plasma membrane rather than in the Golgi. It can reach massive molecular weights (millions of Daltons) and does not attach to a core protein.
Chondroitin & Dermatan Sulfate: These share similar structures but are distinguished by the epimerization of glucuronic acid to iduronic acid in DS. This flexibility allows DS to form unique binding sites for anticoagulants and growth factors.
Heparan Sulfate vs. Heparin: While chemically similar, Heparin is more highly sulfated and primarily stored in mast cells, whereas HS is ubiquitous on cell surfaces and dictates complex signaling events.
Biosynthesis and Biological Function
Biosynthesis Pathways
The biosynthesis of GAGs (excluding HA) occurs primarily in the Golgi apparatus. The process initiates with the formation of a tetrasaccharide linker region (GlcUA-Gal-Gal-Xyl) attached to specific serine residues of the core protein. This step is catalyzed by xylosyltransferases. Following linker formation, specific glycosyltransferases add the characteristic repeating disaccharides.
Subsequent polymer modification is the critical step that generates diversity. A battery of sulfotransferases and epimerases modifies the nascent chain. For instance, in HS synthesis, N-deacetylase/N-sulfotransferase (NDST) enzymes replace acetyl groups with sulfate groups, creating clusters of high sulfation separated by unsulfated regions. This "domain structure" is essential for the specific binding properties of the gag function.
Physiological Functions
- Mechanical Support & Hydration: Due to their high negative charge density, GAGs attract cations and water, creating turgor pressure. This is vital in cartilage (CS) to resist compression and in the skin (HA) for hydration.
- Cell Signaling Regulation: Proteoglycans bearing HS chains act as co-receptors. They bind growth factors like FGF, VEGF, and TGF-beta, protecting them from degradation and presenting them to their high-affinity receptors. This makes the extracellular matrix a dynamic reservoir of signaling molecules.
- Cell Adhesion & Migration: GAGs interact with cell adhesion molecules (e.g., integrins, selectins), modulating cell-matrix interactions essential for embryonic development, leukocyte trafficking, and wound healing.
- Enzyme Regulation: Heparin and HS regulate the activity of proteases and protease inhibitors (e.g., Antithrombin III), playing a central role in the coagulation cascade.
GAG Pathology: Disease Mechanisms
Given their ubiquity and functional importance, defects in GAG synthesis or degradation lead to severe pathological conditions, broadly categorized into lysosomal storage diseases, malignancies, and inflammatory disorders.
Mucopolysaccharidoses (MPS)
MPS are a group of inherited metabolic disorders caused by the deficiency of lysosomal enzymes required to degrade GAGs. This leads to the systemic accumulation of partially degraded GAGs in cells, blood, and connective tissue. Clinical manifestations include skeletal abnormalities, cognitive impairment, and organomegaly. Examples include Hurler syndrome (MPS I) involving dermatan and heparan sulfate accumulation, and Sanfilippo syndrome (MPS III) specifically affecting heparan sulfate degradation.
Cancer and Metastasis
In cancer, the tumor microenvironment often exhibits altered GAG composition. Upregulation of Hyaluronic Acid and specific Chondroitin Sulfate proteoglycans creates a physical barrier that hampers immune cell infiltration. Furthermore, altered sulfation patterns on Heparan Sulfate can promote tumor angiogenesis and metastasis by creating constitutive signaling niches for growth factors like VEGF. Targeting tumor-associated GAGs is a promising therapeutic avenue.
Viral and Bacterial Pathogenesis
Many pathogens exploit cell surface GAGs, particularly Heparan Sulfate, as initial attachment receptors. Viruses such as HSV, HPV, and SARS-CoV-2 bind to HS to concentrate on the cell surface before engaging entry receptors. This interaction is often dependent on specific sulfation motifs, making GAG mimetics potential antiviral candidates.
Inflammation and Atherosclerosis
During inflammation, GAGs like Hyaluronic Acid are fragmented into smaller oligomers which act as Danger-Associated Molecular Patterns (DAMPs), triggering innate immune responses via TLR signaling. In atherosclerosis, increased GAG content in the arterial intima retains LDL cholesterol, initiating plaque formation.
Our Solutions for GAG Research
Investigating the diverse structures and functions of GAGs requires specialized tools. Traditional methods often fail to distinguish between subtle sulfation isomers. Creative Biolabs provides a robust platform for GAG analysis and antibody development.
Glycosaminoglycan (GAG) Microarray
A high-throughput screening tool containing a library of defined GAG oligosaccharides. This platform is ideal for profiling the binding specificity of proteins, antibodies, viruses, and cells against a diverse array of GAG structures including Heparin, HS, CS, DS, and HA.
Tumor-Associated GAG Antibody Development
We develop high-affinity antibodies targeting specific GAG epitopes overexpressed in the tumor microenvironment. These antibodies serve as powerful tools for diagnostic imaging and potential therapeutic targeting of the cancer glycocalyx.
Anti-GAG Sulfation Motif Antibody Development
Precise sulfation patterns dictate GAG function. We offer specialized services to generate antibodies against specific sulfation motifs (neo-epitopes), allowing researchers to map functional domains within complex GAG chains.
Inquire About GAG Services
Published Data1
Understanding the interaction between glycosaminoglycans and pathological agents is central to modern biomedical research. A seminal study published in Biomolecules highlights the critical role of cell surface GAGs in cancer cell biology and treatment. The figure below illustrates the structural diversity of common GAGs, emphasizing the repeating disaccharide units that form the basis for their interaction with signaling molecules and pathogens. This structural complexity underlies the specificity required for viral entry and tumor metastasis.
In the context of viral invasion, Heparan Sulfate proteoglycans (HSPGs) often serve as the initial attachment sites. Research indicates that the sulfation pattern of HS is a determinant factor for the binding affinity of viral envelope proteins. Creative Biolabs utilizes this knowledge to design GAG microarrays that can screen for specific viral-GAG interactions, facilitating the development of entry inhibitors.
FAQs
What is the difference between Glycosaminoglycans (GAGs) and Proteoglycans?
Glycosaminoglycans are long, linear polysaccharide chains. Proteoglycans are macromolecules consisting of a core protein to which one or more GAG chains are covalently attached. Essentially, GAGs are the carbohydrate component of proteoglycans (with the exception of Hyaluronic Acid, which exists as a free polymer).
Why is it difficult to develop antibodies against GAGs?
GAGs are poorly immunogenic because they are self-antigens widely conserved across species. Furthermore, their structural heterogeneity makes it challenging to isolate antibodies that recognize a specific sulfation motif without cross-reactivity. Specialized techniques, such as phage display and unique immunization strategies, are often required.
How do GAGs influence cancer progression?
GAGs influence cancer by modulating the tumor microenvironment. Altered expression of Hyaluronic Acid and Chondroitin Sulfate can increase interstitial pressure and form a protective barrier against immune cells. Additionally, Heparan Sulfate chains can act as reservoirs for growth factors (like VEGF and FGF), promoting angiogenesis and tumor growth.
Can GAGs be used as biomarkers?
Yes. Specific GAG fragments and proteoglycans (like Syndecan-1 or Glypican-3) are shed into the circulation in various diseases, including cancer and sepsis. Detecting specific sulfation patterns or elevated levels of GAGs in serum or urine serves as a potential diagnostic approach for MPS and certain malignancies.
What is the role of the GAG Microarray?
A GAG Microarray allows for the high-throughput analysis of interactions between GAGs and other molecules. It is used to identify the specific GAG structures bound by proteins, antibodies, viruses, or cells, helping to map the "glycome" and identify functional epitopes.
Reference:
- Morla, S. "Glycosaminoglycans and Glycosaminoglycan Mimetics in Cancer and Inflammation." Int. J. Mol. Sci. 2019, 20, 1963; Used under Open Access license CC BY 4.0. https://doi.org/10.3390/ijms20081963
