Tn, STn, and TF Antigens: Conquering the Development Challenges of Tumor-Associated Carbohydrate Antigens (TACAs)
In the intricate world of oncology research, the cell surface is a crowded battlefield. Among the many proteins and lipids vying for attention, a specific class of molecules has emerged as one of the most promising yet challenging targets for immunotherapy: tumor-associated carbohydrate antigens (TACAs). Unlike the stable protein epitopes that defined early vaccine development, TACAs are dynamic, structurally complex, and notoriously difficult to target effectively. The most prominent of these carbohydrate structures—Tn, Sialyl-Tn (STn), and Thomsen-Friedenreich (TF)—are hallmarks of aberrant glycosylation in epithelial cancers. They represent a unique "glycan signature" that distinguishes a tumor cell from its healthy counterpart. However, translating these markers into effective therapies requires overcoming significant hurdles, specifically regarding antigen instability and the generation of specific, high-affinity antibodies.
For researchers navigating this complex landscape, access to precise validation tools is critical. Creative Biolabs supports this frontier of discovery with our comprehensive Anti-MUC1 Glycopeptide Antibody Development Service, enabling scientists to generate and validate antibodies that specifically recognize these elusive carbohydrate targets. Let us guide you to understand the specific challenges associated with the three TACAs—Tn, STn, and TF—and examines how recent chemical engineering strategies and antibody validation techniques are paving the way for the next generation of cancer vaccines.
Three Key TACAs: Defining the Targets
To develop effective immunotherapies, we must first understand the specific molecular targets that appear when cellular glycosylation pathways malfunction. In healthy cells, mucins are heavily glycosylated with long, complex carbohydrate chains. In cancer cells, this process is disrupted, leading to the accumulation of truncated, immature glycan structures directly on the peptide backbone. The three most common and clinically relevant TACAs are:
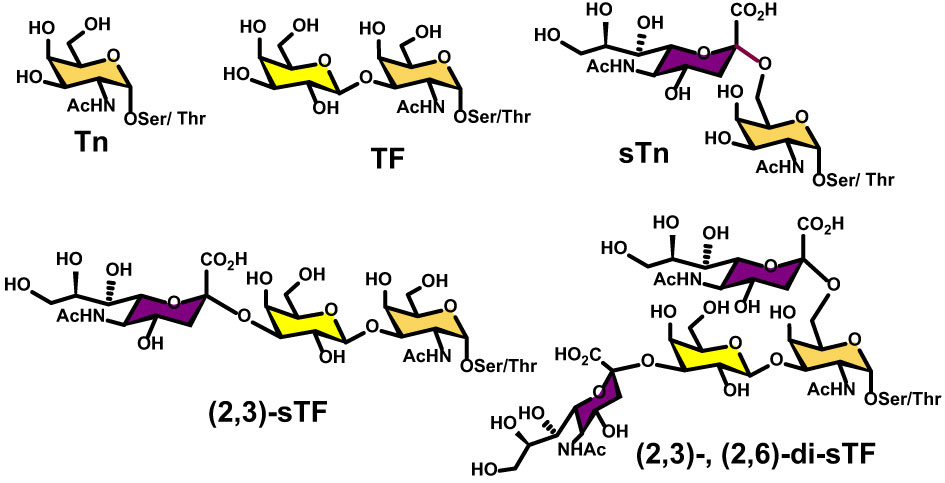 Fig.1 Chemical structures of key tumor-associated carbohydrate antigens (TACAs): Tn, TF, and sTn families.1
Fig.1 Chemical structures of key tumor-associated carbohydrate antigens (TACAs): Tn, TF, and sTn families.1
The Tn Antigen
Structure: GalNAc-Ser/Thr
The Tn antigen is the fundamental unit of this aberrant glycosylation. It is formed by the conjugation of N-acetylgalactosamine (GalNAc) to a serine or threonine residue on the protein backbone.
Significance: It serves as the precursor for other mucin-type O-glycans. In healthy tissue, it is rapidly extended; in tumors, it is often exposed.
Research Focus: Custom Anti-Tn Antibody Development is crucial for identifying early-stage tumorigenesis where this cryptic antigen becomes visible.
The TF Antigen
Structure: Thomsen-Friedenreich
The TF antigen (also known as the T antigen) is formed by the addition of a galactose unit to the Tn antigen (Gal-GalNAc).
Mechanism: Its accumulation is often linked to a deficiency in core 1,3-galactosyl-transferase (T synthase) or mutations in its chaperone, Cosmc.
Research Focus: Custom Anti-T/sT Antibody Development aids in studying cancer cell adhesion and metastasis, where TF expression plays a pivotal role.
The Sialyl-Tn (STn) Antigen
Structure: Neu5Ac-GalNAc
The STn antigen is created when a sialic acid molecule caps the Tn antigen.
Significance: This premature termination prevents the formation of core-2 based glycans, effectively locking the mucin in a hypoglycosylated state. The upregulation of sialyltransferases is a key driver of this phenotype.
Research Focus: Associated with poor prognosis and aggression, Custom Anti-sTn Antibody Development is vital for immuno-oncology research focused on high-risk cancer phenotypes.
The Core Challenge: Antigen Instability and Enzymatic Degradation
While Tn, STn, and TF are ideal targets in theory, using them in practice—specifically in vaccine formulations—presents a formidable biochemical challenge. The literature highlights a critical flaw in using native carbohydrate antigens: bioavailability.
The Enzymatic Barrier
One of the primary reasons vaccines targeting the Tn antigen have historically struggled to maintain efficiency is the sensitivity of the glycan to endogenous enzymes.
- Glycosidase Attack: Native Tn antigens are highly sensitive to endogenous glycosidases present in the body.
- Rapid Degradation: These enzymes break down the TACA structure through enzymatic degradation.
- Consequence: When the carbohydrate component is degraded, the vaccine loses its specific "tumor signature." The immune system may still recognize the peptide carrier, but the critical glycan epitope—the part that distinguishes the tumor from self—is lost, making the antigen less bioavailable in vivo.
This degradation means that even if a vaccine is perfectly designed in the lab, it may fail in the organism simply because the target antigen falls apart before it can induce a robust immune response.
Chemical Engineering Solutions: Mimetics and Analogs
To overcome the inherent instability of natural TACAs, medicinal chemists have turned to structural modification. The goal is to create "mimetics" or "analogs"—structures that look like Tn, STn, or TF to the immune system but are invisible to degradative enzymes.
Fluorinated Analogs
A breakthrough approach detailed in the reviewed literature is the substitution of hydroxyl groups with fluorine atoms. This modification alters the chemical stability of the molecule without significantly changing its 3D shape, allowing it to function as a stable mimic.
- TF Antigen Mimetics: Researchers successfully synthesized TF antigen mimetics by replacing OH groups with two fluorine substituents at the 6 and 6' positions of the pyranose rings. The result was that these fluorinated mimetics generated immune reactions comparable in strength to the native antigen, proving that the immune system accepted the "decoy" as the real target.
- STn Analogs: Similarly, extensive libraries of STn analogs have been synthesized. Yang et al. created 40 different STn analogs, discovering that specific fluorine-containing versions elicited better anti-STn IgG titers than the native structure. Crucially, the antibodies raised against these synthetic analogs were able to detect the native STn antigen on tumor cells.
Clustered Mimetics
Beyond atomic substitution, the spatial arrangement of the antigen matters.
- Clustered Tn: Richichi et al. utilized clustered Tn antigen mimetics to improve stability against glycosidases.
- Outcome: This structural clustering elicited strong IgM and IgG responses (predominantly IgG2a and IgG1) that successfully recognized the native Tn antigens.
These findings underscore a shift in TACA research: the future lies not in isolating natural sugars, but in engineering robust synthetic analogs that can survive the biological environment to trigger potent immunity.
Validation: The Indispensable Role of Specific Antibodies
The development of a stable vaccine construct is only half the battle. The second, equally critical challenge is validation. How do researchers confirm that their vaccine is actually presenting the TACA in a way that the immune system can "see"? It is proven that TACA vaccine's efficacy depends on the quality and specificity of the antibodies it induces.
Proving Glycan-Dependent Cytotoxicity
One of the most significant findings reviewed is the absolute necessity of the glycan for functional immunity.
- The Tn Necessity: In a study of a three-component vaccine, researchers found that a formulation lacking only the Tn antigen (containing only the peptide) failed to produce antibodies capable of Antibody-Dependent Cellular Cytotoxicity (ADCC).
- The Conclusion: This demonstrated conclusively that glycosylation is essential for the antibody to recognize the tumor cell and initiate lysis. Without the specific TACA, the antibody might bind, but it won't kill.
Specificity: Tumor vs. Normal
For TACAs like TF and STn, specificity is paramount. Validating a vaccine candidate requires demonstrating that the induced antibodies bind to tumor cells expressing the aberrant glycan but not to healthy cells.
- Cell Line Validation: Studies consistently used specific cancer cell lines for validation, such as MCF-7 and T47D, which naturally express aberrantly glycosylated MUC1.
- Negative Controls: Success was defined by antibodies that showed strong binding to these tumor lines while showing no binding to non-cancerous human mammary epithelial cells.
This level of validation requires access to high-quality, custom-developed antibodies that can serve as benchmarks and detection tools. Whether assessing a fluorinated STn analog or a clustered Tn vaccine, researchers need control antibodies to verify that the immune response is directed against the specific carbohydrate moiety and not just the linker or carrier protein.
Conclusion: Bridging the Gap with Custom Antibody Development
The field of TACA research has moved beyond simple observation to active engineering. We now understand that Tn, STn, and TF are not just passive markers but unstable targets that require chemical ingenuity to exploit. The transition from native sugars to fluorinated analogs and clustered mimetics represents a major leap forward in overcoming enzymatic degradation and immune tolerance. However, the complexity of these synthetic glycoconjugates demands equally sophisticated validation tools. The ability to distinguish between a successful anti-glycan response and a non-specific background signal is what separates a failed trial from a breakthrough therapy.
At Creative Biolabs, we specialize in the unique challenges posed by carbohydrate antigens. Our expertise extends to the development of high-affinity antibodies against both native TACAs and their synthetic mimetics. Whether you are validating the immunogenicity of a novel fluorinated STn vaccine or seeking to map the expression of TF antigen in metastatic tissue, our custom antibody services provide the precision you need. Don't let antigen instability or lack of specific reagents stall your progress. Partner with Creative Biolabs to develop the custom tools required for your specific glycan targets.
Discuss Your Anti-Glycan Antibody Needs
Reference:
- Roy, René. "Cancer cells and viruses share common glycoepitopes: exciting opportunities toward combined treatments." Frontiers in Immunology (2024): 1292588. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3389/fimmu.2024.1292588
