Anti-MUC16 (CA-125) Glycopeptide Antibody Development Service
MUC16, widely recognized in the clinical setting for its soluble fragment CA-125, is a high-molecular-weight transmembrane mucin that plays a critical role in the pathogenesis of ovarian cancer. While conventional CA-125 antibodies have served as the gold standard for serum biomarkers, the true biological complexity of MUC16 lies in its extensive and often aberrant glycosylation. At Creative Biolabs, we go beyond traditional peptide targeting. We offer a comprehensive service for the development of high-affinity anti-glycopeptide antibodies that specifically recognize the tumor-associated glyco-forms of MUC16. These novel reagents provide researchers with powerful tools for therapeutic discovery, including the development of ADCs and CAR-T candidates, as well as precision biomarker research.
Background: The Complex Landscape of MUC16 and Glycosylation
MUC16 is the largest member of the mucin family, consisting of a heavily glycosylated N-terminal domain, a massive tandem repeat region containing the CA-125 epitopes, and a crucial C-terminal domain (MUC16-CT) that anchors the protein to the cell surface. In healthy tissues, such as the ocular surface and respiratory tract, MUC16 functions as a protective barrier. However, in malignancies such as ovarian high-grade serous carcinoma, pancreatic ductal adenocarcinoma, and breast cancer, MUC16 is significantly overexpressed.
Crucially, the glycosylation machinery in tumor cells is often dysregulated, leading to the expression of truncated O-glycans (such as Tn, sTn, and T antigens) or abnormal N-linked structures on the MUC16 backbone. These "glycopeptide" epitopes—combinations of the specific peptide sequence and the aberrant sugar—represent neo-antigens that are highly specific to cancer cells and virtually absent in normal tissues. For example, the MUC16 glycopeptide antibody strategy allows for the targeting of the membrane-proximal regions that are retained on the tumor cell surface even after the shedding of the CA-125 fragment, offering a stable target for therapeutic research.
The Challenge of Specificity
Developing antibodies against these combined targets is notoriously difficult. The carbohydrate moiety is often weakly immunogenic, and the peptide backbone is often buried or sterically hindered. Furthermore, commercially available anti-CA-125 antibodies typically target the protein backbone within the tandem repeats without regard for glycosylation status, leading to potential cross-reactivity with MUC16 expressed in benign conditions like endometriosis or pregnancy. There is an urgent need for reagents that show high affinity and specific binding to the tumor-exclusive glycopeptide interface. Our platform addresses this gap by utilizing chemo-enzymatic synthesis of complex glycopeptides and sophisticated immunization strategies to break tolerance and generate antibodies with robust validation data.
Comprehensive Development Workflow
Our Specialized Services
We provide a flexible platform tailored to your specific research needs, whether you are targeting the classical CA-125 neoepitope or novel glycosylation sites found in ovarian cancer.
Anti-MUC16 Monoclonal Antibody Development
We utilize advanced hybridoma technology or single B-cell sorting to generate high-affinity monoclonal antibodies. Our focus is on identifying clones that bind specifically to the MUC16 glycopeptide interface, minimizing off-target binding to normal mucins. This service includes humanization and affinity maturation options for therapeutic candidates.
Custom Glycopeptide Antibody Development
This is our flagship service for targeting tumor-specific antigens. We can synthesize MUC16 fragments carrying specific Tn, sTn, or T antigens. Our chemo-enzymatic synthesis platform ensures precise control over the glycosylation site, enabling the generation of antibodies against defined MUC16 glycoforms found in ovarian or pancreatic cancer, such as the glycosylated MUC16 antibody.
Recombinant Antibody Engineering
We can convert successful binders into scFv, Fab, or VHH formats suitable for CAR-T cell engineering or bispecific antibody construction. This is particularly relevant for MUC16, where large IgG molecules may have poor tumor penetration compared to smaller fragments. We can also generate anti-CA-125 neoepitope antibody formats for specific research assays.
Broad Target Capabilities
Beyond MUC16, our expertise extends to a wide range of mucin and glycoprotein targets. We offer development services for anti-MUC1 antibody (including MUC1-Tn, MUC1-STn), anti-MUC4 antibody, anti-MUC5AC antibody, and anti-PODXL antibody (podocalyxin). Whether you need a cancer specific CD43 antibody or an intestinal mucin antibody, our platform can adapt to your target of interest.
Why Choose Our MUC16 Platform?
High Specificity
Our strict counter-screening protocols against naked peptides and irrelevant glycopeptides ensure that our antibodies are truly glycopeptide-specific, reducing false positives in diagnostic assays.
Robust Validation
We provide comprehensive data packages, including flow cytometry on live MUC16+ ovarian cancer cells and IHC on tissue arrays to confirm tumor selectivity.
Diverse Formats
From full IgG to scFv and bispecific formats for therapeutic development, we can engineer the optimal molecule for your specific application.
Expert Support
Our PhD-level scientists provide guidance on epitope mapping, antigen design, and selection of the most relevant cell models for your validation studies.
Applications
- CAR-T Cell Therapy Research: Developing CAR constructs that target the membrane-proximal MUC16 glycoforms retained on the cell surface after CA-125 cleavage. This strategy aims to minimize potential off-target effects and enhance tumor targeting efficiency.
- Antibody-Drug Conjugates (ADCs): Utilizing the rapid internalization properties of MUC16 to deliver cytotoxic payloads specifically to ovarian cancer marker antibody positive cells. Our antibodies are screened for internalization efficiency.
- Biomarker & Diagnostic Research: Creating sensitive reagents for detecting aberrant MUC16 glycosylation (e.g., MUC16-Tn) in serum or tissue biopsies. These tools can serve as potential prognostic indicators or for retrospective analysis of research samples.
- Bispecific Antibodies: Designing T-cell engagers that simultaneously bind the MUC16 glycopeptide on tumor cells and CD3 on T-cells to redirect cytotoxic immunity.
Inquire About MUC16 Antibodies
Published Data
The specificity of monoclonal antibodies to the carboxy-terminus of MUC16 is crucial for evaluating therapeutic candidates, particularly for modalities requiring a stable membrane-bound target. In a pivotal study by Das et al. (2018), researchers developed novel monoclonal antibodies (mAbs) specifically targeting the membrane-retained carboxy-terminal (CT) fragment of MUC16. The data below (Fig. 1) illustrates the generation and characterization of these antibodies. Part A depicts the structure of the MUC16 CT domain, indicating the specific fragment used for immunization, which includes the transmembrane domain and cytoplasmic tail. The binding specificity of selected clones was confirmed via indirect ELISA (Part B) against purified MUC16 CT protein. Furthermore, flow cytometry analysis (Part C) demonstrated that these anti-MUC16 CT mAbs (such as 5E6) effectively bind to MUC16 on the surface of transfected MIA PaCa-2 cells and naturally expressing OVCAR-3 ovarian cancer cells, verifying their ability to recognize the native antigen conformation.
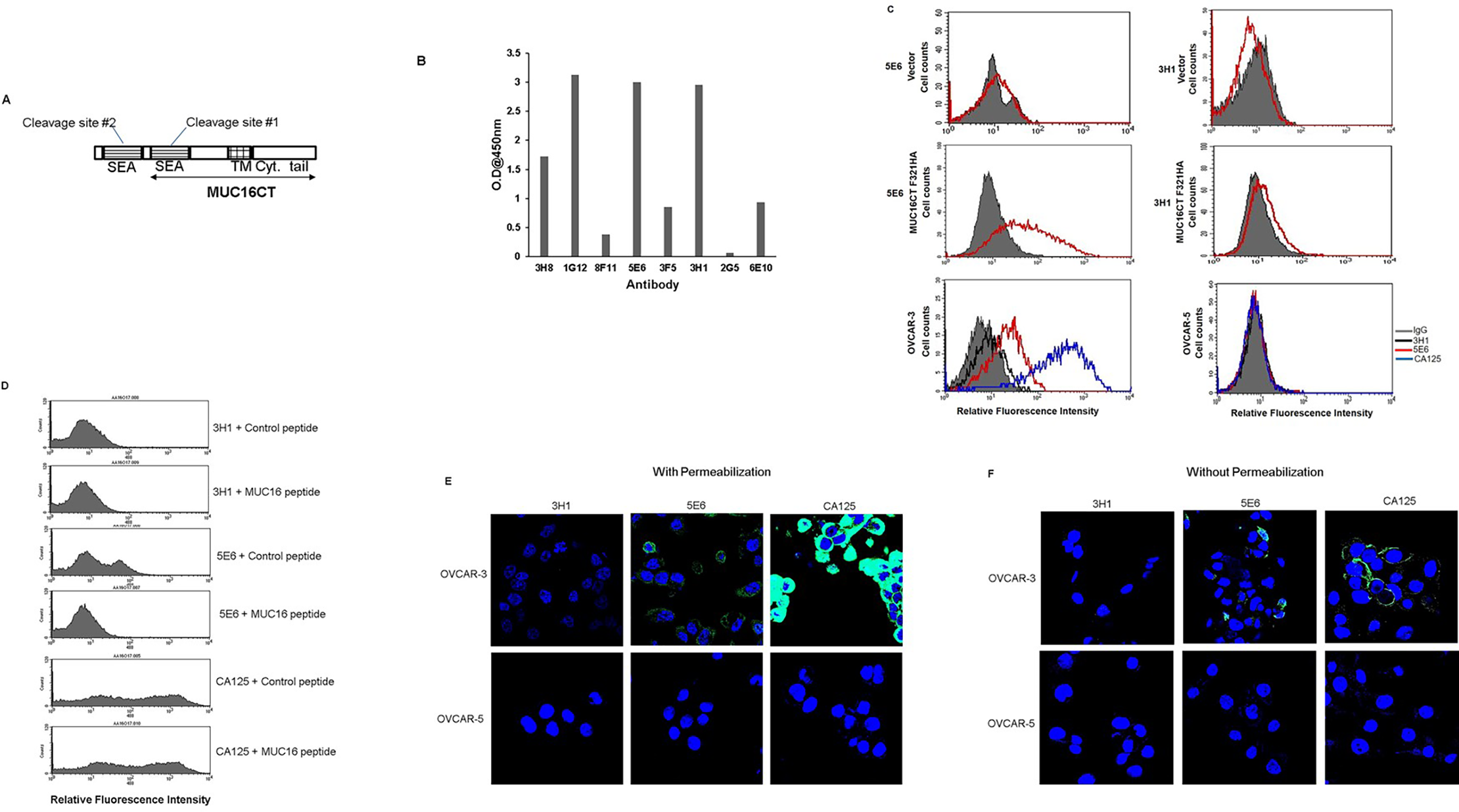 Fig.1 Generation and characterization of monoclonal antibodies (mAbs) to MUC16 C-terminal (CT) domain.1
Fig.1 Generation and characterization of monoclonal antibodies (mAbs) to MUC16 C-terminal (CT) domain.1
FAQs
How do you ensure the antibody targets the glycopeptide and not just the peptide?
We employ a rigorous dual-screening process during the selection phase. Positive selection is performed against the synthesized glycosylated MUC16 peptide (e.g., MUC16-Tn or MUC16-sTn). Negative selection is then conducted against the naked peptide and irrelevant glycopeptides. Only clones that bind the MUC16 glycopeptide significantly better than the controls are selected for further development, ensuring glyco-specificity.
Do you offer humanized antibodies for preclinical research?
Yes, we provide comprehensive antibody humanization services (CDR grafting) to reduce immunogenicity for downstream preclinical development. Furthermore, we can generate fully human antibodies from the start using transgenic mouse platforms or human phage display libraries, accelerating your discovery timeline.
What type of validation data is provided with the antibodies?
Our standard validation package includes ELISA data showing binding affinity (EC50) and specificity. For project-specific needs, we offer Western Blotting, Flow Cytometry (FACS) on relevant cell lines (e.g., OVCAR-3 vs. negative controls), and Immunohistochemistry (IHC) on tissue microarrays.
Can you target other mucins besides MUC16?
Absolutely. Our platform is versatile and has been successfully used to develop antibodies against a wide range of mucins and glycoproteins, including anti-MUC1 antibody, anti-MUC4 antibody, anti-MUC5AC antibody, and anti-PODXL antibody. We can target specific aberrant glycoforms for any of these proteins, such as MUC1-Tn or tumor-specific CD43.
What is the typical timeline for a custom glycopeptide antibody project?
A typical project, from antigen synthesis to the delivery of purified hybridoma supernatant or recombinant antibody, takes approximately 4 to 6 months. This timeline can vary depending on the complexity of the antigen, the host species selected, and the extent of the validation studies required. We provide a detailed Gantt chart and regular progress updates throughout the project.
Reference:
- Das, S.; et al. Development and characterization of carboxy-terminus specific monoclonal antibodies for understanding MUC16 cleavage in human ovarian cancer. PLOS ONE. 2018, 13(3): e0193907. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1371/journal.pone.0193907
Supports
- Anti-MUC1 Glycopeptide Antibody Development
- Anti-MUC4 Glycopeptide Antibody Development
- Anti-MUC16 (CA-125) Glycopeptide Antibody Development
- Anti-MUC5AC Glycopeptide Antibody Development
- Anti-MUC2 Glycopeptide Antibody Development
- Anti-Podocalyxin (PODXL) Glycopeptide Antibody Development
- Anti-CD43 Glycopeptide Antibody Development
- Custom Glycopeptide Target Antibody Development
