Lipoteichoic Acid (LTA) in Gram-Positive Bacteria
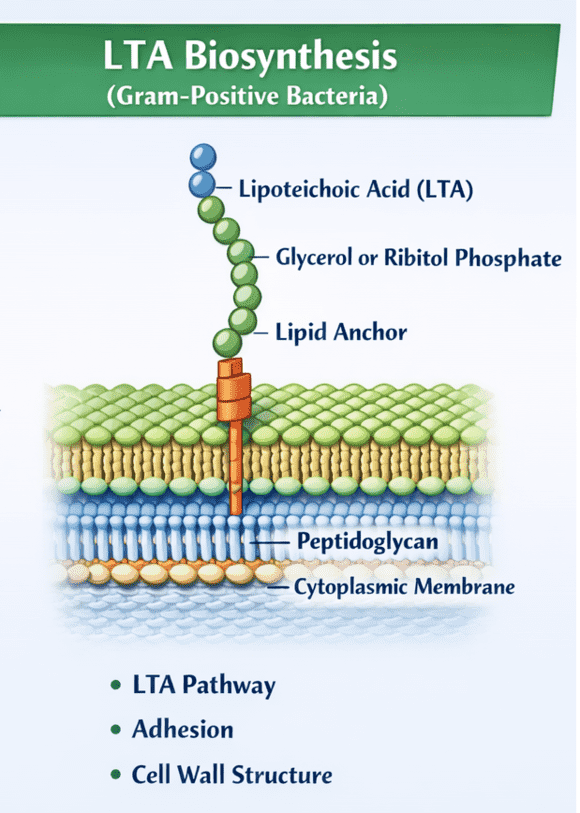
The Gram-positive cell envelope is a complex, dynamic armor that defines the interface between the bacterium and its environment. Embedded within the thick meshwork of peptidoglycan are various glycopolymers, the most prominent of which are teichoic acids. Creative Biolabs offers specialized research tools to study these critical molecules, including our Anti-Lipoteichoic Acid (LTA) Antibody Development service. Understanding the structural nuances and physiological roles of LTA is essential for unravelling the mechanisms of bacterial adhesion, biofilm formation, and host immune recognition.
The Architecture of the Gram-Positive Cell Wall
The hallmark of Gram-positive bacteria is a substantial cell wall composed of multiple layers of peptidoglycan. This structure provides osmotic protection and determines cell shape. However, the cell wall is not merely a rigid exoskeleton; it is threaded with anionic glycopolymers known as teichoic acids. These molecules are classified into two distinct types based on their anchoring mechanism:
- Wall Teichoic Acid (WTA): These are covalently linked to the N-acetylmuramic acid residues of the peptidoglycan layers. They extend outward but originate from within the wall matrix.
- Lipoteichoic Acid (LTA): In contrast, LTA is an amphiphilic molecule anchored directly to the bacterial cell membrane via a glycolipid moiety. Its poly-glycerol phosphate chains extend vertically through the peptidoglycan layers and protrude onto the cell surface.
Together, LTA and WTA create a continuum of negative charge across the cell envelope, termed the "gram-positive cell wall" ion-exchange matrix. This anionic environment is critical for enzyme function, cell division, and interaction with the host environment.
Structural Complexity of Lipoteichoic Acid
The LTA structure is highly variable among different species, yet it generally follows a conserved architectural theme consisting of a glycolipid anchor and a hydrophilic backbone.
The Glycolipid Anchor
The hydrophobic anchor is typically a glycolipid, such as diglucosyl-diacylglycerol (Glc2-DAG) in Staphylococcus aureus or a galactofuranosyl-containing lipid in other species. This lipid moiety inserts into the outer leaflet of the cytoplasmic membrane, securing the polymer. The variability in the fatty acid composition of this anchor can influence the fluidity of the membrane and the dynamic movement of the LTA polymer.
The Poly-Glycerol Phosphate (PGP) Backbone
Extending from the anchor is a long chain of repeating glycerol phosphate units (Type I LTA). The C2 position of the glycerol residues is often modified with D-alanine esters or glycosyl residues (such as N-acetylglucosamine or glucose).
- D-Alanylation: The addition of positively charged D-alanine groups reduces the net negative charge of the polymer. This modification is tightly regulated by the dlt operon and is crucial for resistance to cationic antimicrobial peptides (CAMPs).
- Glycosylation: Sugar substituents on the backbone act as specific antigenic determinants and receptors for bacteriophages. These modifications are key targets for serological typing and antibody development.
Physiological Functions of LTA
The lipoteichoic acid function extends far beyond structural integrity. It is a central regulator of bacterial physiology and survival.
Cation Homeostasis
The phosphate groups in the LTA backbone provide a high density of negative charges, allowing the cell wall to function as a reservoir for divalent cations, particularly magnesium (Mg2+). By sequestering Mg2+, LTA ensures that essential membrane-bound enzymes have access to this cofactor, even in magnesium-limiting environments.
Regulation of Autolysins
Bacterial growth requires the precise cleavage of peptidoglycan to allow for cell wall expansion and daughter cell separation. Autolysins (Muramidases) are the enzymes responsible for this cleavage. LTA acts as a spatial and temporal inhibitor of these enzymes, preventing uncontrolled cell lysis. The degree of D-alanylation on LTA modulates this inhibition, thereby fine-tuning cell division.
Bacterial Adhesion and Biofilm Formation
LTA is a potent adhesin. Its amphiphilic nature and protruding structure allow it to mediate bacterial adhesion to host tissues. LTA binds to fibronectin on epithelial cells, facilitating the initial colonization step during infection. Furthermore, the release of D-alanine from LTA has been implicated in the maturation and detachment phases of biofilm formation, influencing the persistence of chronic infections on medical devices.
Immunobiology: The Inflammatory Response
In the context of host-pathogen interactions, LTA is frequently referred to as the "endotoxin of Gram-positive bacteria," functionally analogous to Lipopolysaccharide (LPS) in Gram-negatives. It is a potent Pathogen-Associated Molecular Pattern (PAMP).
When shed from the bacterial surface—either during cell division or induced by antibiotic treatment—LTA triggers a robust innate immune response. The recognition mechanism involves a complex of host receptors:
- LBP and CD14: Lipopolysaccharide-binding protein (LBP) transfers LTA to membrane-bound CD14 on monocytes and macrophages.
- TLR2 Heterodimerization: CD14 presents LTA to Toll-like Receptor 2 (TLR2), which heterodimerizes with TLR6 (typically) or TLR1 to form a signaling complex.
- Signaling Cascade: This interaction recruits MyD88 and activates NF-κB and MAP kinases.
The result is the massive release of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) and chemokines (IL-8). While this inflammatory response is intended to clear the infection, excessive activation by high loads of LTA can lead to systemic inflammatory response syndrome (SIRS) and septic shock.
Our Solutions for LTA Research
Targeting LTA is challenging due to its amphiphilic nature and propensity to form micelles in solution. Creative Biolabs has developed optimized protocols to generate high-affinity reagents against this complex glycolipid.
Anti-LTA Antibody Development
We produce monoclonal and polyclonal antibodies that specifically recognize the LTA backbone or the glycolipid anchor. Our immunization strategies involve the use of whole-bacteria or LTA-carrier protein conjugates to ensure proper antigen presentation. These antibodies are validated for use in ELISA, flow cytometry, and inhibition assays to block LTA-induced inflammation.
Microbial Glycan Microarray
To study the specificity of LTA-binding proteins or serum antibodies, we offer high-throughput microbial glycan arrays. These arrays contain purified LTA variants from diverse species (e.g., S. aureus, S. pyogenes, B. subtilis), allowing for rapid profiling of cross-reactivity and epitope mapping.
Custom Glycosylation Services
For researchers needing defined LTA structures, we provide chemical and enzymatic synthesis services. We can generate synthetic LTA analogs with specific D-alanine or glycosyl substitution patterns to investigate structure-function relationships in a controlled setting.
Glycosylation Analysis
Precise structural characterization of LTA extracted from clinical isolates. We utilize NMR and Mass Spectrometry (MS) to determine chain length, acylation patterns, and the degree of substitution, providing a complete structural profile of your target molecule.
Inquire about LTA Solutions
Published Data
Recent research highlights the critical role of LTA in tumor progression, particularly in non-small-cell lung cancer (NSCLC). A study demonstrated that purified LTA from Staphylococcus aureus significantly promotes the proliferation of A549 and H226 lung cancer cells. This pro-proliferative effect is dose-dependent and mediated through the activation of Toll-like Receptor 2 (TLR-2). Upon binding, LTA triggers the release of Interleukin-8 (IL-8), which acts in an autocrine or paracrine manner to stimulate cell growth. Crucially, the use of neutralizing antibodies against either TLR-2 or IL-8 was shown to completely abrogate this LTA-induced proliferation, confirming the specificity of the pathway. These findings underscore the potential of targeting the LTA-TLR2-IL8 axis in infection-associated lung malignancies.
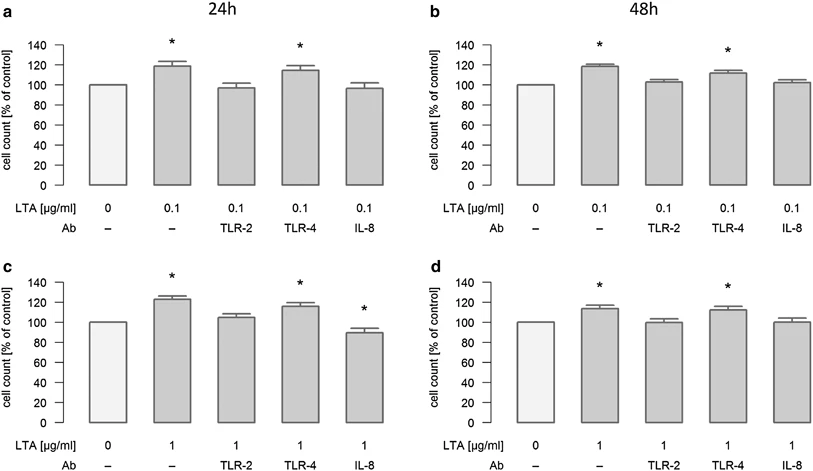 Fig.2 Neutralization of lta induced proliferation in lung cancer cells.1
Fig.2 Neutralization of lta induced proliferation in lung cancer cells.1
FAQs
What is the main difference between LTA and LPS in terms of immune activation?
While both are potent immunostimulants, they signal through different Toll-like Receptors. LPS (from Gram-negatives) is recognized by the TLR4/MD-2 complex. LTA (from Gram-positives) is recognized by TLR2, typically as a heterodimer with TLR6 (or sometimes TLR1). LTA generally requires higher concentrations to elicit a cytokine storm comparable to LPS.
Why is LTA considered difficult to work with in immunological assays?
LTA is amphiphilic, possessing both a hydrophilic phosphate backbone and a hydrophobic lipid anchor. In aqueous solutions, purified LTA tends to form micelles, which can mask epitopes and alter its bioactivity. Critical micelle concentration (CMC) effects must be considered when designing ELISA or stimulation assays.
Does the structure of LTA vary significantly between bacterial species?
Yes. While the general architecture is conserved, the composition of the glycolipid anchor, the length of the PGP chain, and the nature of the substituents (D-alanine vs. glycosyl groups) vary widely. For example, Streptococcus pneumoniae contains phosphorylcholine substituents on its teichoic acids, which are absent in Staphylococcus aureus.
Can Creative Biolabs generate antibodies that distinguish LTA from WTA?
Yes. Although LTA and WTA often share similar poly-glycerol phosphate backbones, they differ in their anchoring mechanisms and often in their specific glycosylation patterns. We use counter-screening strategies and specific antigen design (including the glycolipid anchor for LTA) to select clones that show minimal cross-reactivity with Wall Teichoic Acid.
What is the role of D-alanine in LTA function?
D-alanine esterification introduces positive charges to the LTA backbone, reducing its overall net negative charge. This is a resistance mechanism against cationic antimicrobial peptides (defensins) produced by the host, which target negatively charged bacterial surfaces. It also modulates the binding of autolysins and divalent cations.
Supports
- Anti-Bacterial Gram-Positive Glycan (LTA) Antibody Development
- Microbial Glycan Antigen Microarray
- Glycosylation Analysis Services
Reference:
- Hattar, Katja, et al. "Lipoteichoic acids from Staphylococcus aureus stimulate proliferation of human non-small-cell lung cancer cells in vitro." Cancer Immunology, Immunotherapy (2017): 799-809. Distributed under Open Access license CC BY 4.0. https://doi.org/10.1007/s00262-017-1980-4
