The Role of Hyaluronic Acid (HA) in Tumor Microenvironment
Hyaluronic acid (HA), a non-sulfated glycosaminoglycan (GAG) distributed throughout the extracellular matrix (ECM), plays a dual role in physiology and pathology. While essential for tissue homeostasis, hydration, and mechanical integrity, its aberrant accumulation is a hallmark of many solid tumors, often termed "hyaluronic acid cancer" association. This accumulation fundamentally reshapes the tumor microenvironment (TME), influencing cancer cell proliferation, invasion, metastasis, and multidrug resistance. Creative Biolabs leverages deep expertise in glycan biology to provide comprehensive research solutions, including Anti-Hyaluronic Acid (HA) Antibody Development, to decipher the complex biology of HA in oncology.
HA Biology and the Tumor Microenvironment Architecture
The role of HA in the tumor microenvironment is determined largely by its molecular weight and local concentration, creating a physical and biochemical niche that supports malignancy.
The Paradox of Molecular Weight
HA exhibits a dichotomous nature based on polymer length. High molecular weight HA (HMW-HA) generally maintains tissue integrity and suppresses angiogenesis. In contrast, in the TME, rapid turnover mediated by hyaluronidases (HYALs) and reactive oxygen species generates low molecular weight HA (LMW-HA) fragments. These fragments act as "danger signals," triggering pro-inflammatory cytokines and stimulating angiogenesis, thereby fueling tumor progression.
Interstitial Fluid Pressure (IFP) and Drug Resistance
Due to its high negative charge and hydrophilicity, HA traps water molecules, causing significant swelling of the ECM. In the confined space of a tumor, this creates high Interstitial Fluid Pressure (IFP). This elevated pressure collapses tumor blood vessels, leading to hypoxia and creating a physical barrier that prevents chemotherapeutic agents and immune cells from penetrating the tumor core. This biophysical mechanism is a primary driver of resistance to standard therapies in HA-rich tumors like pancreatic ductal adenocarcinoma (PDAC).
Synthesis and Turnover Regulation
HA concentration is regulated by the balance between its synthesis by Hyaluronan Synthases (HAS1, HAS2, HAS3) and degradation by Hyaluronidases (HYAL1, HYAL2). In the TME, oncogenic signaling (e.g., KRAS, PI3K) often upregulates HAS2 in both carcinoma cells and Cancer-Associated Fibroblasts (CAFs), leading to the excessive deposition of a dense HA matrix. This matrix not only supports tumor structure but also facilitates the sequestration of growth factors.
CD44 Signaling: The Core Axis of Malignancy
The interaction between HA and its cell surface receptors is crucial for translating the extracellular glycan signal into intracellular responses. The primary receptor, CD44, acts as a "cd44 signaling" hub that coordinates adhesion, migration, and survival.
| Receptor | Key Function in Cancer | Signaling Consequence |
|---|---|---|
| CD44 (Standard & Variants) | Primary HA receptor; Stem cell marker (CSC) | Activates PI3K/Akt and RAS/MAPK pathways; promotes cytoskeletal reorganization via Ezrin/Radixin/Moesin (ERM). |
| RHAMM (CD168) | Motility and mitosis regulation | Cooperates with CD44 to sustain RAS signaling; critical for centrosome integrity and spindle assembly during division. |
| LYVE-1 | Lymphatic vessel binding | Facilitates lymphatic metastasis by docking tumor cells to the lymphatic endothelium. |
| TLR4 | Innate immune sensing | Recognizes LMW-HA fragments, triggering NF-κB activation and inflammatory cytokine release (IL-6, IL-8). |
Binding of HA to CD44 induces conformational changes that recruit cytoplasmic adaptors, linking the cell surface to the actin cytoskeleton. This linkage drives the formation of filopodia and lamellipodia, essential machinery for cancer cell migration. Furthermore, CD44 signaling supports the "stemness" of cancer cells, contributing to tumor recurrence.
HA in Cancer Metastasis and Invasion
The "ha tumor microenvironment" is not static; it actively facilitates the spread of disease. A HA-rich pericellular coat surrounds aggressive cancer cells, protecting them from immune surveillance and mechanical stress during dissemination.
Epithelial-Mesenchymal Transition (EMT)
High expression of HAS2 and CD44 is strongly correlated with the Epithelial-Mesenchymal Transition (EMT). HA-CD44 interaction activates Twist and Snail transcription factors, repressing E-cadherin and upregulating N-cadherin. This switch allows cells to detach from the primary tumor and acquire a motile, invasive phenotype, a critical step in cancer metastasis.
Angiogenesis and Lymphangiogenesis
LMW-HA fragments are potent angiogenic factors. By interacting with endothelial cells, they stimulate proliferation and tube formation, ensuring the tumor receives the blood supply necessary for growth and providing routes for hematogenous spread.
Targeting HA: Drug Delivery and Therapy
The unique properties of HA in the TME have led to two distinct therapeutic strategies: targeting HA to destroy the tumor niche, and using HA as a vehicle for HA-based drug delivery.
Depleting the Barrier
Enzymatic depletion of HA using PEGylated hyaluronidases (PEGPH20) has been explored to lower interstitial fluid pressure. This "stromal remodeling" strategy aims to reopen collapsed vessels, allowing standard chemotherapies (like gemcitabine) and immune checkpoint inhibitors to penetrate the tumor more effectively.
HA-Based Drug Delivery
Since many tumors overexpress CD44, HA can be used as a targeting ligand. Nanoparticles coated with HA or drug-HA conjugates can selectively bind to CD44-positive tumor cells. Upon binding, the complex is internalized via endocytosis, releasing the cytotoxic payload directly inside the cancer cell while sparing healthy tissues lacking CD44 overexpression.
Inquire About HA Research Solutions
Our Services for HA Research
Creative Biolabs offers a suite of advanced services to support your investigation into the role of HA in the TME.
Anti-Hyaluronic Acid (HA) Antibody Development
Development of specific antibodies (IgG, IgM) and antibody fragments (scFv, VHH) targeting HA or HA-binding proteins. We offer screening strategies to differentiate between HA oligomers and high molecular weight polymers, essential for dissecting their distinct biological roles.
Glycoarray Platforms
High-throughput screening using glycan arrays containing defined HA fragments of various lengths. This platform allows for the rapid identification of HA-binding proteins, receptors, and the characterization of antibody specificity against complex glycan structures.
Glycosylation Analysis
Comprehensive analysis of TME glycosylation patterns. We utilize mass spectrometry and HPLC to profile the glycome of tumor tissues, quantify HA levels, and analyze the expression of HAS and HYAL enzymes in your samples.
Custom Glycosylation of Biomolecules
Enzymatic or chemical synthesis of defined HA polymers and conjugation services. We can generate biotinylated, fluorescently labeled, or drug-conjugated HA probes for imaging studies and the development of HA-based drug delivery systems.
Published Data
A comprehensive review published in Cancers (2024) provides an intricate analysis of the crosstalk between Hyaluronic Acid (HA) and HA-interacting molecules (HAIMs) within the tumor microenvironment (TME). The study elucidates how the aberrant accumulation of HA, particularly its low molecular weight fragments, orchestrates a pro-tumorigenic niche by engaging with specific cell surface receptors such as CD44 and RHAMM. This receptor-ligand interaction triggers a cascade of critical intracellular signaling pathways, most notably the RAS/RAF/MEK/ERK and PI3K/Akt axes, which are fundamental drivers of cancer cell proliferation, survival, and epithelial-mesenchymal transition (EMT). Furthermore, the review highlights the structural role of secreted HAIMs, such as Versican and TSG-6, in remodeling the extracellular matrix architecture to create a hydrated, permissive path for tumor invasion and angiogenesis. The authors also examine the complex influence of HA on immune evasion, noting that specific HA-protein complexes can modulate the recruitment and function of tumor-associated macrophages and T-cells. These findings underscores the significant prognostic value of HAIM expression profiles across various solid tumors and reinforce the therapeutic potential of disrupting the HA-CD44 signaling axis to effectively dismantle the supportive microenvironment required for primary tumor expansion and distant metastasis.
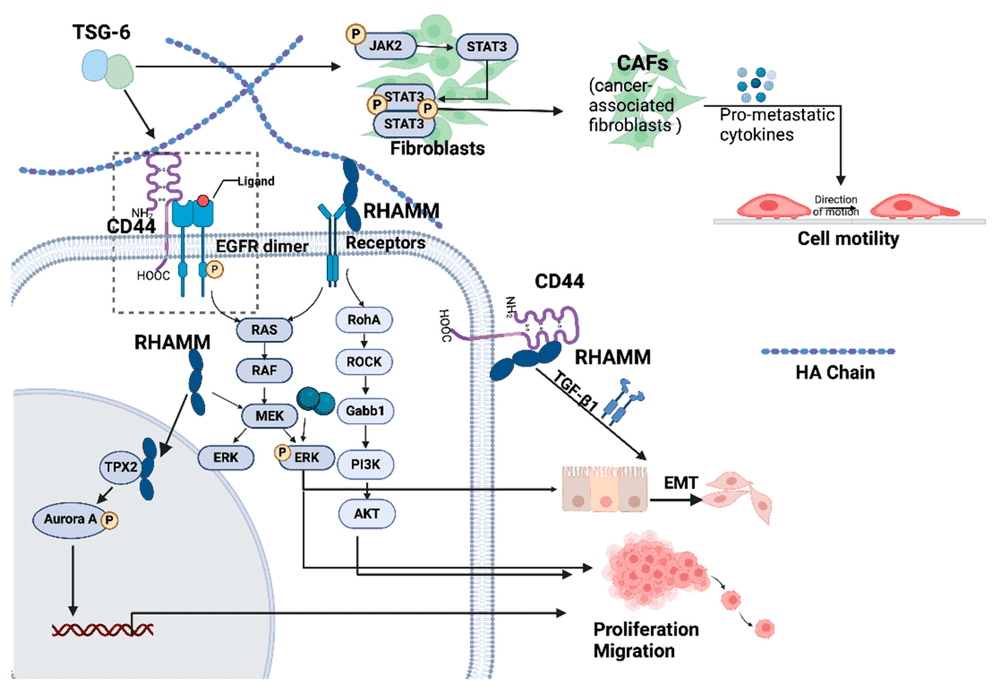 Fig.1
Mechanisms of HA-CD44-RHAMM Signaling in Tumor Progression.1
Fig.1
Mechanisms of HA-CD44-RHAMM Signaling in Tumor Progression.1
FAQs
How does Hyaluronic Acid contribute to drug resistance in tumors?
HA accumulates in the tumor stroma, binding large amounts of water and increasing interstitial fluid pressure (IFP). This high pressure collapses blood vessels, preventing chemotherapy drugs from reaching cancer cells. Additionally, HA-CD44 signaling can upregulate multidrug resistance (MDR) transporters.
What is the difference between HMW-HA and LMW-HA in cancer?
High Molecular Weight HA (HMW-HA) is generally anti-angiogenic and suppresses immune response. However, in the TME, it is degraded into Low Molecular Weight HA (LMW-HA), which is highly active, promoting inflammation, angiogenesis, and tumor invasion.
Why is CD44 considered a key target in HA research?
CD44 is the primary receptor for HA and is often overexpressed on cancer stem cells. The HA-CD44 interaction drives "cd44 signaling" pathways that support self-renewal, metastasis, and survival, making it a critical axis for therapeutic intervention.
Can Creative Biolabs develop antibodies specific to HA?
Yes. While HA is poorly immunogenic, we utilize specialized phage display and immunization strategies to generate antibodies that can recognize specific HA structures or HA-binding proteins, overcoming the challenges of traditional methods.
How acts HA as a vehicle for drug delivery?
"HA-based drug delivery" exploits the overexpression of CD44 on tumor cells. Drugs conjugated to HA or encapsulated in HA-nanoparticles are selectively taken up by these cells via receptor-mediated endocytosis, enhancing efficacy and reducing systemic toxicity.
Reference:
- Xu, Y.; Benedikt, J.; Ye, L. "Hyaluronic Acid Interacting Molecules Mediated Crosstalk between Cancer Cells and Microenvironment from Primary Tumour to Distant Metastasis." Cancers 16.10 (2024): 1907. Distributed under Open Access license CC BY 4.0. https://doi.org/10.3390/cancers16101907
