Anti-Bacterial (Gram-Positive) Glycan & LTA Antibody Development Service
The cell envelope of Gram-positive bacteria represents a formidable, multi-layered barrier that is fundamental to bacterial physiology, cell division, and interaction with the host immune system. Unlike Gram-negative organisms, which possess an outer membrane targeted by our Anti-Bacterial (Gram-Negative) Glycan & LPS Antibody Development Service, Gram-positive bacteria display a thick meshwork of peptidoglycan that is densely functionalized with unique anionic glycopolymers known as teichoic acids. These structures, specifically Lipoteichoic Acid (LTA) and Wall Teichoic Acid (WTA), act as critical surface antigens and virulence factors. At Creative Biolabs, we have established a sophisticated platform dedicated to the generation of high-affinity antibodies against these complex carbohydrate and glycolipid targets. Our specialized service overcomes the inherent low immunogenicity of bacterial glycans to deliver precise LTA antibody, peptidoglycan antibody, and capsule antibody reagents. These research tools are essential for characterizing surface topology, investigating antibiotic resistance mechanisms (such as methicillin resistance in Staphylococcus aureus), and developing robust immunological assays for pathogen detection.
Navigating the Complex Landscape of Gram-Positive Surface Glycans
The surface of Gram-positive bacteria is a rich landscape of carbohydrate targets that are accessible to the extracellular environment. This accessibility makes them prime candidates for detection biomarkers and potential targets for research investigation, yet their structural complexity requires a nuanced approach for antibody development. The three primary classes of surface glycans include amphiphilic lipoteichoic acids, covalently anchored wall teichoic acids, and the underlying peptidoglycan matrix. Each of these components plays a distinct role in bacterial survival and interaction with the host environment.
Lipoteichoic Acid (LTA): The Amphiphilic Anchor
LTA is a macro-amphiphile anchored to the bacterial cell membrane via a glycolipid moiety, with a long polyglycerol phosphate backbone extending through the peptidoglycan layers to the cell surface. This molecule is ubiquitous among Gram-positive bacteria and plays a key role in regulating autolysins and cell division by controlling the cation homeostasis of the cell envelope. Because the glycerol phosphate backbone is highly conserved, an anti-lipoteichoic acid antibody often serves as a broad-spectrum Gram-positive marker. However, species-specific variations exist, particularly in the D-alanine esters and glycosyl substituents (such as N-acetylglucosamine or galactose) attached to the backbone. These subtle modifications can be leveraged to generate more specific reagents, such as a staphylococcus aureus LTA antibody, which can distinguish between pathogenic strains and commensal flora based on epitope density and structure. Furthermore, LTA is a potent immunomodulator, stimulating Toll-like receptor 2 (TLR2) and triggering inflammatory cascades, making anti-LTA antibodies critical for studying sepsis models and host immune responses.
Wall Teichoic Acid (WTA): The Covalent Regulator
Chemically distinct from LTA, Wall Teichoic Acid is covalently linked to the N-acetylmuramic acid residues of the peptidoglycan via a conserved linkage unit (Man-GlcNAc-phosphate). In many pathogens like Staphylococcus aureus, the WTA backbone consists of polyribitol phosphate, which is heavily modified with glycosyl residues. These modifications are not random; they are enzymatically driven (e.g., by TarM or TarS glycosyltransferases) and dictate critical biological interactions, including bacteriophage binding and resistance to host antimicrobial peptides. A specific WTA antibody is a powerful tool for studying these modifications. For instance, the beta-O-GlcNAc modification on WTA has been directly implicated in methicillin resistance, making anti-WTA antibodies vital for research into antibiotic resistance mechanisms and biofilm formation. Unlike LTA, WTA is exclusively found in the cell wall and is not anchored in the membrane, providing a distinct spatial target for antibody binding.
Peptidoglycan and Capsular Polysaccharides
The peptidoglycan layer provides the essential structural integrity of the cell. It is composed of alternating N-acetylglucosamine (GlcNAc) and N-acetylmuramic acid (MurNAc) sugar chains cross-linked by short peptides. An anti-peptidoglycan antibody can be designed to recognize the glycan backbone, which is generally conserved, or specific peptide stem structures that may vary between species (e.g., Lys-type vs. DAP-type peptidoglycan). Beyond the cell wall, many virulent pathogens, such as Streptococcus pneumoniae and Group B Streptococcus, are encapsulated by a polysaccharide capsule. This capsule acts as a cloak against phagocytosis. Generating a polysaccharide capsule antibody or a pneumococcal polysaccharide antibody is critical for serotyping, epidemiological surveillance, and assessing the efficacy of glycoconjugate vaccine candidates in preclinical models.
Challenges in Developing Antibodies Against Cell Wall Glycans
Generating high-affinity antibodies against bacterial carbohydrates presents a unique set of challenges that differ significantly from protein targets. These hurdles often lead to the failure of standard immunization protocols, necessitating specialized strategies to achieve success.
T-Cell Independence and Low Immunogenicity
Most bacterial polysaccharides, including LTA and peptidoglycan, are T-cell independent type 2 antigens. They typically induce a short-lived IgM response with little to no affinity maturation or isotype switching to IgG in the host. This makes it difficult to raise high-affinity IgG antibodies using conventional immunization. We overcome this by employing advanced carrier protein conjugation techniques (such as reductive amination or CDAP activation) to link glycan haptens to immunogenic carriers like CRM197 or KLH, effectively recruiting T-cell help and driving a robust IgG response.
Structural Complexity and Zwitterionic Motifs
Some capsular polysaccharides and cell wall glycans possess zwitterionic charge motifs that are critical for immune activation but can be lost during purification or chemical synthesis. Maintaining the native conformation and charge distribution of the antigen is essential for generating functional antibodies. Our synthesis platform utilizes precise chemical steps to ensure that these delicate structural features, including labile O-acetyl groups and phosphate bridges, are preserved in the final immunogen construct.
Cross-Reactivity and Contamination
Extracting glycans from bacterial cells often results in contamination with other cell wall components, such as Protein A in Staphylococcus aureus, which binds immunoglobulins non-specifically via the Fc region and interferes with screening. Furthermore, the conserved backbones of LTA and WTA can lead to cross-reactivity between related species. We utilize highly purified synthetic glycans and rigorous counter-selection strategies against closely related strains to eliminate cross-reactive clones early in the screening process.
Epitope Accessibility and Masking
In the native cell wall, deeper layers of peptidoglycan or membrane-proximal regions of LTA may be masked by the capsule or surface proteins. Antibodies generated against purified components may fail to bind whole bacteria due to steric hindrance. Our validation workflow includes binding assays on live, encapsulated bacteria and cell wall-defective mutants to ensure that the selected clones recognize surface-accessible epitopes that are relevant for in vivo applications.
Comprehensive Anti-Gram-Positive Glycan Antibody Services
Creative Biolabs provides a modular, end-to-end service tailored to the unique biochemistry of Gram-positive cell walls. We handle the entire pipeline, from the precision synthesis of complex glycan haptens to the purification and characterization of functional antibodies.
Custom Anti-LTA Monoclonal Antibody Development
We specialize in the development of antibodies targeting the conserved glycerol phosphate backbone or specific D-alanine/glycosyl substitutions of LTA. Our anti-LTA monoclonal antibody service utilizes both purified native LTA (extracted using methods that preserve the lipid anchor) and defined synthetic analogues conjugated to immunogenic carriers. This approach allows us to generate reagents capable of neutralizing inflammatory responses driven by TLR2 activation or detecting broad categories of Gram-positive bacteria. We can optimize screening to achieve staphylococcus aureus LTA antibody specificity or broader cross-reactivity against Bacillus and Enterococcus species for pan-Gram-positive detection assays. We also offer specific screening against LTA from Group B Streptococcus (GBS) to aid in neonatal sepsis research.
Anti-Wall Teichoic Acid (WTA) Antibody Discovery
Given the critical role of Wall Teichoic Acids in bacterial adherence and host colonization, we provide dedicated wall teichoic acid antibody development services. We focus on targeting the specific glycoforms found in bacterial isolates, such as the alpha-GlcNAc versus beta-GlcNAc modifications on the polyribitol phosphate backbone of S. aureus. These antibodies are invaluable for elucidating the mechanisms of phage infection, biofilm formation, and methicillin resistance. Our platform can discriminate between these subtle structural differences, providing researchers with precise tools to map the glycan landscape of the bacterial surface. We also develop reagents against WTA from Listeria monocytogenes and other foodborne pathogens to assist in detection assay development.
Anti-Peptidoglycan Antibody Engineering
The peptidoglycan mesh is a universal feature of the bacterial cell wall, yet its detection is often complicated by its heterogeneous structure. Our anti-peptidoglycan antibody products are designed to bind either the non-variable glycan strands or specific peptide stems (e.g., Lys-type vs. DAP-type peptidoglycan). These reagents serve as excellent controls for cell wall integrity studies and can be used to monitor bacterial lysis, cell division processes, and the localization of cell wall synthesis machinery in real-time microscopy experiments. Furthermore, we can generate antibodies against peptidoglycan fragments (muropeptides) to study their role as NOD1/NOD2 agonists in innate immune signaling.
Capsular Polysaccharide Antibody Generation
For encapsulated pathogens, the polysaccharide capsule is the primary virulence factor and shield against phagocytosis. We specialize in polysaccharide capsule antibody generation for diverse serotypes. Whether you require a streptococcus pneumoniae capsule antibody for Quellung reactions and serotyping assays, or a group A streptococcus carbohydrate antibody for rapid detection tests, our platform ensures high serotype specificity. We employ rigorous adsorption protocols to remove antibodies against common cell wall components, ensuring that the final reagent is specific to the capsular polysaccharide. We cover a wide range of serotypes for pneumococcus (PCV13, PPSV23 targets) and meningococcus.
Gram-Positive Glycan Antibody Development Workflow
Request a Quote for Your Target
Technical Highlights
Precise Epitope Mapping
Differentiating between subtle modifications, such as D-alanylation levels in LTA or specific glycosylation patterns in WTA using defined glycan arrays.
Gram-Positive Optimized
Protocols specifically tailored for extraction and presentation of antigens from thick cell walls, ensuring native conformation and epitope accessibility.
Synthetic Library Support
Access to a vast library of synthetic glycan fragments for counter-selection, affinity maturation, and fine specificity testing against related structures.
Versatile Formats
Production of full-length IgG, IgM, or recombinant fragments (scFv, Fab) suitable for various research applications and detection kit development.
Applications of Anti-Bacterial Glycan Antibodies in Research
MRSA & Antibiotic Resistance Mechanisms
The structure of WTA is intricately linked to cell wall physiology and antibiotic resistance. Specific glycosyl modifications, such as the beta-O-GlcNAcylation in MRSA, are essential for the expression of methicillin resistance phenotypes. A high-specificity WTA antibody allows researchers to monitor the expression of these resistance-related glycan epitopes, study the regulation of the tarS and tarM genes, and evaluate the efficacy of novel cell-wall targeting antibiotics that disrupt these pathways. These antibodies can also be used to visualize cell wall turnover and synthesis dynamics in real time.
Detection Assay Development
Surface glycans like LTA and capsular polysaccharides are shed by bacteria during infection and can accumulate in body fluids. High-affinity anti-LTA antibody pairs are essential for developing sensitive sandwich ELISAs or lateral flow assays to detect bacteremia caused by S. aureus or Streptococcus species directly from complex biological samples. Our reagents are rigorously validated for these applications to ensure low background and high sensitivity, enabling the rapid identification of pathogens in laboratory settings.
Functional Opsonophagocytic Assays (OPKA)
To assess the functional activity of vaccine research candidates, the Opsonophagocytic Killing Assay (OPKA) is the gold standard. We provide pneumococcal polysaccharide antibody and other anti-capsule reagents that serve as positive controls or reference standards in these assays. These antibodies facilitate the measurement of opsonophagocytic titers, helping to validate the immunogenicity and protective potential of new glycoconjugate vaccine candidates in preclinical models. They are also essential for serotyping bacterial isolates in epidemiological studies.
Host-Pathogen Interaction Studies
Cell wall glycans are potent pathogen-associated molecular patterns (PAMPs) that interact with host pattern recognition receptors (PRRs) such as TLR2 and various lectins. Anti-peptidoglycan antibody and bacillus subtilis cell wall antibody reagents are widely used to block these interactions in vitro. This allows researchers to dissect the specific signaling pathways involved in the innate immune response and determine the contribution of individual cell wall components to inflammation and pathogenesis. This is crucial for understanding the mechanisms of sepsis and immune evasion.
Published Data
The structural diversity of Wall Teichoic Acid (WTA) in Staphylococcus aureus presents a significant challenge for antibody recognition, yet understanding this diversity is key to developing effective therapeutics. A landmark study published in ACS Central Science utilized a library of fully defined synthetic WTA fragments to elucidate the molecular binding mechanisms of human monoclonal antibodies (mAbs) to specific WTA glycoforms. The research focused on discriminating between alpha-GlcNAc and beta-GlcNAc modifications, the latter of which is often associated with methicillin-resistant strains. X-ray crystallography analysis revealed that specific mAbs, such as clone 4497, recognize a unique conformational epitope formed by the beta-GlcNAc residue and the ribitol phosphate backbone. These antibodies demonstrated the ability to bind with high affinity to the bacterial surface, effectively distinguishing between the different glycoforms. This specificity is crucial for research applications, as the beta-form is often dominant in MRSA isolates and is involved in resistance mechanisms. The research highlights the potential of using defined glycan antigens to raise precise staphylococcus aureus LTA antibody and WTA-targeting reagents for immunotherapy research and mechanistic studies.
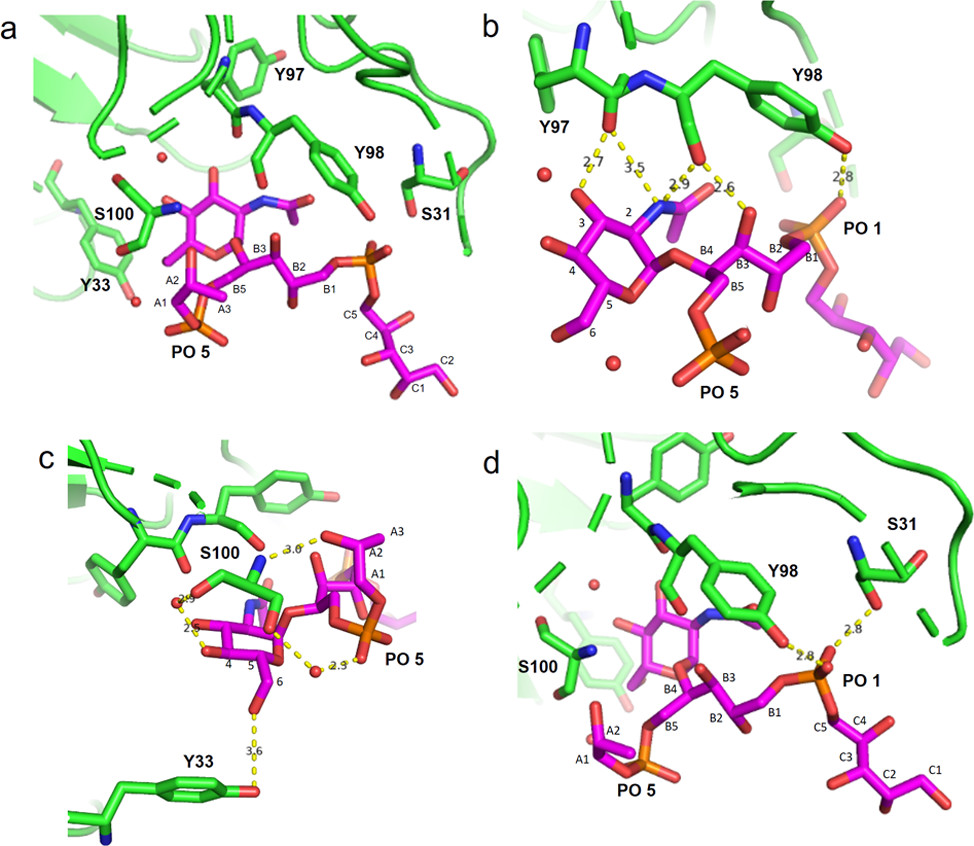 Fig.1 Structural basis of specific recognition of S. aureus WTA glycoforms by monoclonal antibodies.1
Fig.1 Structural basis of specific recognition of S. aureus WTA glycoforms by monoclonal antibodies.1
FAQs
How do you ensure your anti-LTA antibody does not cross-react with mammalian DNA or phospholipids?
Both LTA and DNA share a phosphate-rich backbone, which can historically lead to cross-reactivity issues with some polyclonal preparations. To prevent this, we employ a rigorous counter-screening process during the hybridoma selection phase. We screen clones against mammalian DNA, phospholipids, and unrelated phosphate-containing polymers. Furthermore, our immunization strategy focuses on the unique glycolipid anchor structure or species-specific glycosyl substitutions (like GlcNAc or D-Ala) to ensure the anti-lipoteichoic acid antibody is highly specific to the bacterial target and does not bind host tissues.
Can you generate antibodies specific to MRSA Wall Teichoic Acid modifications?
Yes, absolutely. MRSA strains often exhibit specific and distinct glycosylation patterns on their WTA, most notably the beta-O-GlcNAcylation mediated by the TarS enzyme. By using synthetic WTA fragments that represent these specific beta-O-GlcNAc epitopes as immunogens, we can generate a WTA antibody that preferentially binds to MRSA strains over other staphylococci that may primarily express the alpha-form. This specificity is confirmed using glycan microarrays and binding assays on characterized knockout or overexpression bacterial strains.
Do you offer antibodies for Bacillus subtilis cell wall research?
Yes, we provide specialized bacillus subtilis cell wall antibody development services. Bacillus subtilis possesses a unique WTA structure, often based on polyglycerol phosphate in certain strains (like strain 168) or polyribitol phosphate in others (like strain W23). We can design antigens to target these specific structures, allowing for precise localization studies in this important Gram-positive model organism. These antibodies are particularly useful for studies on cell division, morphogenesis, and cell wall turnover.
What is the best antibody format for detecting Peptidoglycan in various assays?
The optimal format depends on your specific application. For the detection of bacterial lysis, general staining, or Western blotting, a high-affinity IgG format is the standard recommendation due to its stability and ease of use. However, peptidoglycan is a highly repetitive antigen. For applications like agglutination assays or when studying particulate antigens, an IgM format anti-peptidoglycan antibody can offer higher avidity, leading to stronger binding and aggregation. We can produce both formats, as well as recombinant scFv or Fab fragments, depending on your experimental needs.
Reference:
- Soliman, C., et al. "Antibody Recognition of Different Staphylococcus aureus Wall Teichoic Acid Glycoforms." ACS Central Science 8.9 (2022): 1345-1358. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1021/acscentsci.2c00125
