Targeting Aberrantly Glycosylated MUC1: A Precision Strategy for Cancer Immunotherapy
In the complex landscape of cancer immunotherapy, the distinction between "self" and "non-self" is the battleground where therapeutic success is determined. Among the myriad of potential targets, MUC1 (Mucin 1) stands out as a high-priority antigen for translational research. However, MUC1 poses a unique paradox: it is a protein expressed abundantly on normal epithelial tissues, from the lungs to the pancreas. The key to utilizing MUC1 as a safe and effective target lies not in the protein sequence itself, but in its coat. The differentiating factor is glycosylation. In healthy cells, MUC1 is adorned with elaborate carbohydrate chains. In tumor cells, this architecture collapses, resulting in aberrantly glycosylated MUC1 that exposes unique antigenic structures. For researchers and drug developers, understanding this structural divergence is critical. It shifts the focus from simple protein targeting to the precise recognition of glycopeptides.
At Creative Biolabs, we understand that targeting these complex neoantigens requires sophisticated tools. Our Anti-MUC1 Glycopeptide Antibody Development Service is designed to assist researchers in generating high-affinity antibodies that can distinguish between the tumor-specific "naked" MUC1 and its healthy, masked counterpart.
The Structural Divergence: Hyperglycosylated vs. Hypoglycosylated
To appreciate the challenge of targeting MUC1, one must first visualize its architecture. MUC1 is a transmembrane protein consisting of an intracellular C-terminal region and a large extracellular N-terminal domain. This extracellular domain is characterized by a Variable Number of Tandem Repeat (VNTR) units, each consisting of 20 amino acids.
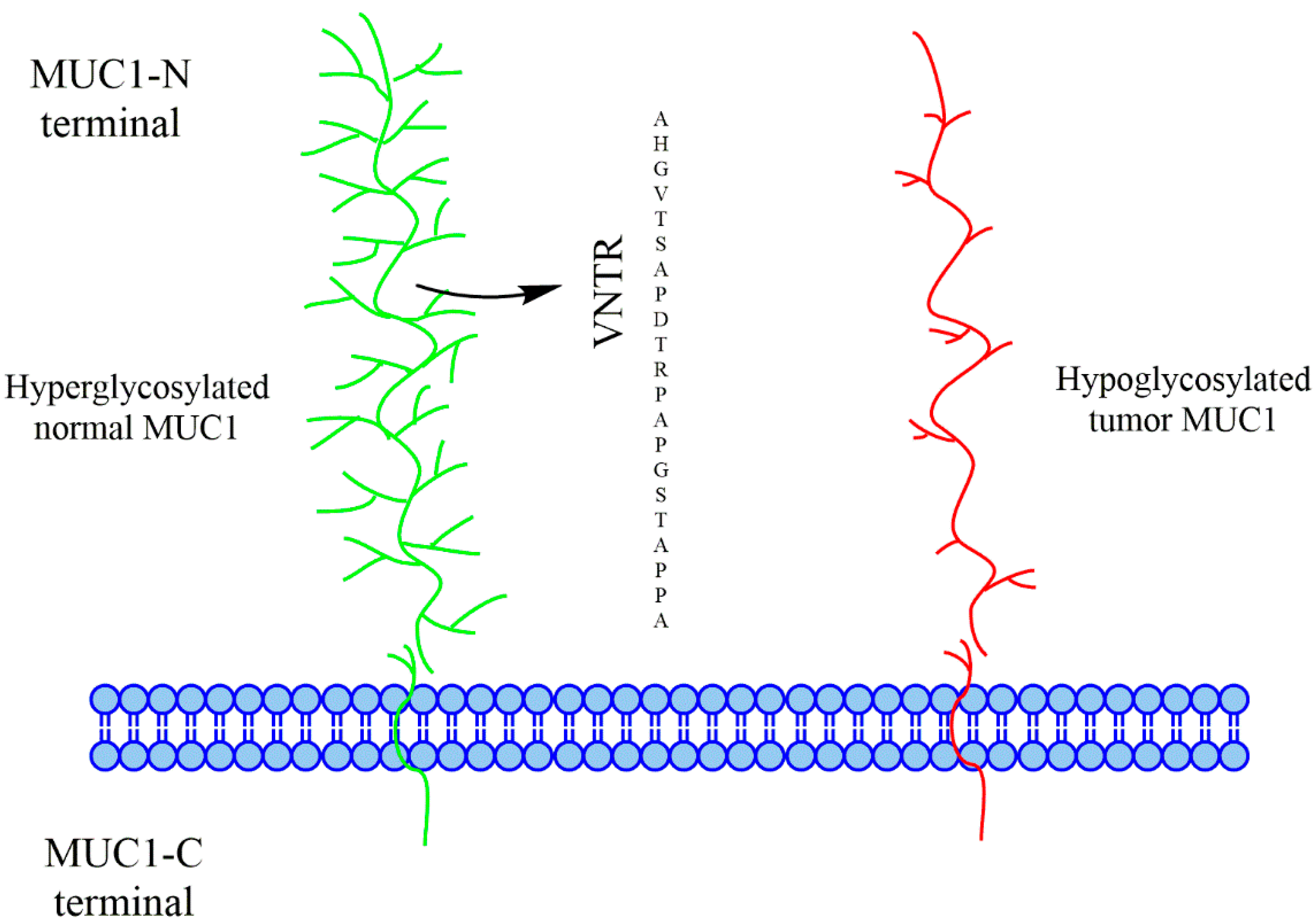
Fig.1 Glycosylation patterns in normal vs. tumor-associated MUC1.1
The Protective Shield of Normal Cells
In normal epithelial cells, the VNTR region is the site of extensive O-linked glycosylation. Each repeat contains five potential sites (serine or threonine residues) where carbohydrate chains can attach.
- Hyperglycosylation: Normal MUC1 is "hyperglycosylated," meaning it carries long, branched carbohydrate chains.
- The Masking Effect: These extensive glycans act as a physical shield, masking the peptide backbone of the VNTR. This prevents the immune system from accessing the core peptide epitopes, effectively maintaining tolerance.
The Exposure in Tumor Cells
In carcinoma cells, this glycosylation machinery malfunctions. The result is tumor-associated MUC1 that is hypoglycosylated or aberrantly glycosylated.
- Hypoglycosylation: The carbohydrate chains are drastically shortened.
- Peptide Exposure: Because the sugar chains are truncated, the peptide backbone of the VNTR—previously hidden—becomes exposed to the immune system.
This structural difference shows normal MUC1 is a dense, bottle-brush structure, while tumor MUC1 appears "naked" or sparsely decorated. This exposure creates a dual-specificity target: the immune system can potentially recognize the newly exposed peptide and the truncated carbohydrates simultaneously. This specific combination is what makes MUC1 an attractive target for cancer immunotherapy.
Unmasking the TACAs: Tn, STn, and TF Antigens
The aberrant glycosylation of MUC1 is not random; it follows specific patterns driven by the dysregulation of cellular enzymes. The incomplete synthesis of glycans leads to the expression of specific tumor-associated carbohydrate antigens (TACAs) directly on the MUC1 peptide backbone.
The Mechanism of Aberrance
The appearance of these truncated antigens is primarily due to the malfunction of glycosyltransferases in the Golgi apparatus:
Enzyme Deficiency
Tumors are often deficient in core 1,3-galactosyl-transferase (T synthase), an enzyme required to elongate the sugar chain.
Premature Termination
Upregulation of sialyltransferases attaches a sialic acid cap to the nascent sugar chain too early, preventing the formation of complex core-2 based glycans.
Chaperone Mutation
Mutations in the Cosmc chaperone, which is required for T synthase activity, also lead to these truncated structures.
The "Big Three" MUC1 Antigens
Tn Antigen
Formed by the conjugation of N-acetylgalactosamine (GalNAc) to a serine or threonine residue. It is the simplest O-glycan and a hallmark of cancer.
TF Antigen
Thomsen-Friedenreich antigen. Formed by the addition of galactose to the Tn antigen (Gal-GalNAc).
Sialyl-Tn (STn)
Formed when a sialic acid is added to the Tn antigen (Neu5Ac-GalNAc). This prevents further elongation and is strongly associated with poor prognosis and metastasis.
These carbohydrate structures do not exist in isolation; they are chemically linked to the MUC1 peptide. Therefore, an effective therapeutic agent must often recognize the glycopeptide unit—the sugar plus the amino acid context—rather than the sugar or peptide alone.
The Glycopeptide Imperative in Immunotherapy Design
For decades, the field of anti-MUC1 antibody development and vaccine design struggled with a fundamental issue: immunologic tolerance. Because MUC1 is a "self-antigen" present on normal cells, the immune system is educated to ignore it.
The Failure of Non-Glycosylated Approaches
Early attempts to vaccinate against MUC1 utilized non-glycosylated peptides. These attempts largely failed to induce effective anti-tumor immunity.
- Structural Mismatch: Antibodies raised against naked, synthetic peptides did not recognize the native, glycosylated MUC1 found on tumor cells.
- Lack of Specificity: The immune system generated antibodies for a linear peptide sequence, but on the tumor, that sequence interacts with truncated sugars (Tn, STn), altering its conformation.
The Problem with Heavy Glycosylation
Conversely, using fully glycosylated MUC1 (mimicking normal tissue) is also ineffective. These structures are heavily processed and often induce tolerance or are simply not processed efficiently by antigen-presenting cells.
The Solution: Targeting the Aberrant Glycopeptide
The ideal target for cancer immunotherapy is the specific combination of the MUC1 VNTR peptide and the tumor-specific truncated glycans.
- Proof of Concept: Research indicates that vaccines containing the Tn antigen (GalNAc) induce antibodies capable of Antibody-Dependent Cellular Cytotoxicity (ADCC). However, when the Tn antigen was removed (leaving only the peptide), the resulting antibodies could not initiate tumor lysis.
- Crucial Conclusion: This finding proves that the presence of the specific glycan (Tn) is absolutely essential for the antibody to recognize the tumor cell and trigger its destruction.
Validating Efficacy: The Role of Specific Antibodies
In the development of MUC1 glycopeptide cancer vaccines, the primary measure of success is the quality of the antibodies produced. It is not enough to simply generate a high titer of IgG; the antibodies must be biologically active and highly specific to the tumor form of MUC1.
Differentiating Tumor from Normal
The ultimate validation of a vaccine construct is its ability to generate antibodies that bind to specific cancer cell lines, such as MCF-7 (breast cancer) or T47D.
- MCF-7 & T47D: These cell lines express the aberrantly glycosylated MUC1 naturally. Binding to these cells confirms that the vaccine-induced antibodies can recognize the native antigen configuration.
- Negative Controls: Crucially, successful antibody development requires demonstrating a lack of binding to non-cancerous human mammary epithelial cells. This ensures that the therapy will not target healthy tissues, reducing the risk of autoimmune side effects.
The Importance of IgG Subtypes
Th1 vs. Th2 Bias
The generation of IgG2a and IgG2b subtypes (in mice) is often cited as evidence of a Th1-type response, which is associated with effective cellular immunity and tumor clearance.
Mechanism of Action
These subtypes are particularly effective at recruiting immune effector cells to the tumor site. Vaccines using gold nanoparticles (AuNP) as carriers were able to induce these favorable IgG subtypes even at lower antigen concentrations, suggesting more efficient presentation of the glycopeptide structure.
Synthetic Precision
The trend in MUC1 research is moving toward fully synthetic vaccines where every component—the peptide sequence, the specific glycan (Tn, STn, TF), and the adjuvant—is chemically defined.
Case Study
Researchers synthesized MUC1 glycopeptides conjugated to T-helper epitopes. The resulting antibodies showed strong binding to T47D cells, validating that the synthetic glycopeptide accurately mimicked the surface of the cancer cell. Furthermore, vaccines lacking the specific carbohydrate component failed to induce ADCC, reinforcing that the glycopeptide is the functional unit of recognition.
Conclusion: The Future is Glycopeptide-Specific
The scientific literature confirms that MUC1 is a premier target for cancer treatment, but only if the target is defined with structural precision. The "naked" peptide is insufficient, and the "normal" glycoprotein is protected. The therapeutic window exists solely within the aberrantly glycosylated MUC1—the unique interface of truncated sugars (Tn, STn, TF) and the VNTR peptide backbone.
For drug developers, this means that the screening and validation of antibodies must be rigorous. Tools must be developed that specifically recognize these glycopeptide epitopes without cross-reacting with the heavily glycosylated MUC1 on vital organs. The move toward synthetic glycoconjugates represents the maturation of this field, offering the ability to engineer vaccines and therapies that strike the tumor where it is most vulnerable: its structural deformity.
Explore our Anti-MUC1 Antibody Solutions
Reference:
- Apostolopoulos, John M., Geoffrey A. Pietersz, and Vasso Apostolopoulos. "MUC1 Glycopeptide Vaccines: Clinical Status and Future Prospects." Vaccines (2016): 25. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3390/vaccines4030025
