Heparan Sulfate vs. Heparin: Structural Differences and Biological Roles
Glycosaminoglycans (GAGs) are a family of complex, linear polysaccharides that play critical roles in cell signaling, matrix assembly, and tissue homeostasis. Among these, heparin and heparan sulfate (HS) are frequently confused due to their structural similarities and shared biosynthetic pathways. While both are comprised of repeating disaccharide units of glucosamine and uronic acid, they differ fundamentally in their sulfation patterns, cellular localization, and primary biological functions. At Creative Biolabs, we specialize in distinguishing these subtle structural nuances to support precision research. Our comprehensive platform, including anti-heparan sulfate antibody development, enables researchers to dissect the specific roles of HSPGs in development and disease.
Biosynthesis and Localization: The First Point of Divergence
The distinction between heparin and heparan sulfate begins at the cellular level. Heparin is synthesized exclusively in the specialized secretory granules of connective tissue mast cells. It functions primarily as a stored product, released only upon degranulation during immune or allergic responses. In contrast, heparan sulfate is a ubiquitous component of the cell surface and extracellular matrix (ECM) of virtually all animal cells. It exists as heparan sulfate proteoglycans (HSPGs), such as syndecans and glypicans, which are anchored to the cell membrane, or perlecan, which is secreted into the basement membrane.
This difference in location dictates their structural evolution. Heparin is processed to be exceptionally uniform and highly sulfated to maximize charge density for packing within granules. Heparan sulfate, however, retains a high degree of structural heterogeneity to serve as a versatile "co-receptor" or "docking site" for a vast array of signaling molecules, morphogens, and enzymes in the extracellular environment.
Structural Comparison: Domain Organization and Sulfation
The heparan sulfate structure is characterized by a unique domain organization that is absent in heparin. HS chains are polymers of alternating glucosamine and uronic acid residues. However, modifications to these sugars occur in clusters.
- NA-domains: Regions rich in N-acetylated glucosamine (GlcNAc) and glucuronic acid (GlcA). These regions have low sulfation and provide flexibility to the chain.
- NS-domains: Highly sulfated regions rich in N-sulfated glucosamine (GlcNS) and iduronic acid (IdoA). These domains are the primary binding sites for protein ligands.
- SAS-domains: Transition zones containing alternating N-acetylated and N-sulfated residues.
In contrast, heparin lacks this segmented architecture. It can be viewed as consisting almost entirely of extended NS-domains, resulting in a uniformly high negative charge density. The heparin difference lies in this hypersulfation: while heparin is continuously modified, heparan sulfate maintains a "code" of sulfated and non-sulfated regions that dictates specific protein interactions.
| Feature | Heparin | Heparan Sulfate (HS) |
|---|---|---|
| Cellular Source | Mast cells (secretory granules) | Ubiquitous (cell surfaces, ECM) |
| Domain Structure | Continuous high sulfation | Distinct domains (NA, NS, SAS) |
| N-Sulfation | High (>80%) | Variable (typically 40-50%) |
| Iduronic Acid (IdoA) | Major uronic acid (>70%) | Variable, often < 50% (GlcA rich) |
| Primary Function | Anticoagulation, defense | Cell signaling, morphogen gradients, cell adhesion |
Functional Implications: Anticoagulation vs. Signaling
Anticoagulation: The Heparin Signature
The clinical utility of heparin relies on a specific pentasaccharide sequence containing a rare 3-O-sulfated glucosamine. This sequence binds to Antithrombin III (ATIII) with high affinity, inducing a conformational change that accelerates the inhibition of thrombin and Factor Xa. While this sequence can occur in heparan sulfate, it is much less frequent. Consequently, heparin is a potent anticoagulant, whereas native cell-surface HS exhibits only weak anticoagulant activity under physiological conditions.
FGF Binding and HSPG Signaling
Heparan sulfate's primary role is orchestrating signaling complexes. A prime example is the FGF binding mechanism. Fibroblast Growth Factors (FGFs) require HS as a cofactor to bind their receptors (FGFRs) effectively. The specific sulfation patterns within the NS-domains of HS facilitate the dimerization of FGF-FGFR complexes, triggering downstream tyrosine kinase signaling. This hspg signaling capability is tunable; different cells can modify the sulfation pattern of their surface HS to alter their sensitivity to growth factors, thereby regulating developmental processes like limb bud formation and angiogenesis.
Our Solutions for Glycan Research
Distinguishing between these highly related structures requires specialized tools. Creative Biolabs offers a suite of services designed to analyze, detect, and target specific GAG structures.
Anti-Heparan Sulfate Antibody Development
We generate antibodies capable of distinguishing specific sulfation motifs on heparan sulfate chains. These tools are essential for mapping the distribution of "active" HS structures in tissues without cross-reacting with general heparin.
Heparan Sulfate Microarray
Our high-throughput microarray platform allows researchers to screen protein interactions against a library of defined HS oligosaccharides. This is ideal for identifying the specific binding requirements of growth factors, viral proteins, or antibodies.
Glycosylation Analysis
We provide comprehensive structural characterization of GAGs, including disaccharide composition analysis and sequencing, to quantify the "heparin difference" in your samples.
Inquire About GAG Comparison Services
Published Data
A comprehensive editorial review (2019) highlights the critical structural and biosynthetic distinctions between heparin and heparan sulfate (HS), clarifying why these chemically related glycosaminoglycans perform such distinct biological functions. Although they originate from the same biosynthetic precursors, their mature architectures diverge significantly due to cell-specific enzymatic regulation. The study details how heparin is synthesized primarily in mast cells as an exceptionally highly sulfated, relatively uniform polymer, optimized for dense packaging and potent anticoagulant activity. In contrast, heparan sulfate serves as a ubiquitous cell-surface regulator characterized by a sophisticated domain organization. Unlike the continuous sulfation of heparin, HS chains act as information-rich scaffolds, containing specific clusters of modified disaccharides (S-domains) separated by flexible, non-sulfated regions (NA-domains). This segmented architecture allows HS to present precise binding motifs for diverse signaling proteins, such as growth factors, rather than functioning simply as a highly charged interactor. The authors emphasize that while heparin is frequently used as a convenient experimental proxy, it lacks the complex domain spacing and structural plasticity of native HS required for orchestrating sophisticated cellular processes.
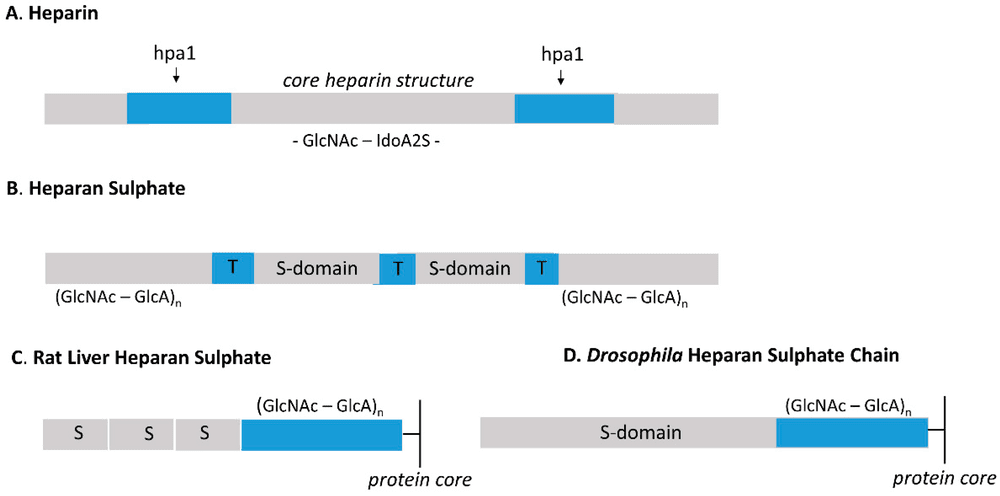 Fig.1
Schematic comparison of the molecular design and domain organization in Heparin versus Heparan Sulfate.1
Fig.1
Schematic comparison of the molecular design and domain organization in Heparin versus Heparan Sulfate.1
FAQs
Why is heparin used as an anticoagulant instead of heparan sulfate?
Heparin contains a much higher concentration of the specific pentasaccharide sequence required to bind and activate Antithrombin III. While heparan sulfate can contain this sequence, it is rare and dispersed, making HS a poor anticoagulant in comparison.
Can antibodies differentiate between heparin and heparan sulfate?
Yes, but it requires careful selection. Many antibodies, such as the widely used clone 10E4, recognize specific N-sulfated/N-acetylated motifs common in HS. Other antibodies are generated to target hyper-sulfated regions more characteristic of heparin. Specificity validation using glycan arrays is critical.
What is the role of the NA-domain in heparan sulfate?
The NA-domains (N-acetylated) are flexible, low-sulfation regions that separate the highly sulfated NS-domains. They are thought to act as spacers, ensuring the correct spatial presentation of ligand-binding domains to cell surface receptors.
Does FGF bind to heparin or heparan sulfate?
In a physiological context, FGF binds to cellular heparan sulfate. However, in laboratory settings, heparin is often used as a high-affinity substitute because its high sulfation mimics the binding sites found in the NS-domains of HS.
How does sulfation pattern affect function?
The sulfation pattern (positions 2-O, 6-O, and 3-O) creates a specific "barcode." For example, 6-O-sulfation is critical for FGF signaling, while 3-O-sulfation is essential for antithrombin binding and Herpes Simplex Virus entry.
Reference:
- Yates, E. A., J. T. Gallagher, and M. Guerrini. "Introduction to the Molecules Special Edition Entitled 'Heparan Sulfate and Heparin: Challenges and Controversies': Some Outstanding Questions in Heparan Sulfate and Heparin Research." Molecules 24.7 (2019): 1399. Distributed under Open Access license CC BY 4.0. https://doi.org/10.3390/molecules24071399
