Herpesvirus Glycoproteins and Immune Modulation
Herpesviruses, including Herpes Simplex Virus (HSV) and Cytomegalovirus (CMV), are ubiquitous pathogens that have co-evolved with their hosts for millions of years. Central to their success is a sophisticated arsenal of surface glycoproteins that orchestrate viral entry and manipulate the host immune response. These glycoproteins are heavily glycosylated, presenting a dense array of N-linked and O-linked glycans that serve not only as structural shields but also as functional ligands for cellular receptors. At Creative Biolabs, we understand that dissecting the nuances of anti-herpesvirus glycan antibody development is critical for advancing next-generation therapeutics and vaccines. Our specialized platform provides researchers with high-affinity tools to investigate these complex carbohydrate-protein interactions.
Introduction to Herpesvirus Glycoprotein
The Herpesviridae family is characterized by a large, double-stranded DNA genome encased in an icosahedral capsid, surrounded by a proteinaceous tegument and a lipid envelope. Embedded within this envelope are numerous viral glycoproteins, which are the primary determinants of infectivity and antigenicity. Unlike simple viruses that may rely on a single protein for attachment and fusion, herpesviruses utilize a multi-component machinery.
These glycoproteins do not function in isolation. They form complex higher-order structures that are essential for the viral life cycle. For instance, the hsv entry mechanism requires the coordinated action of four key glycoproteins: gD, gB, and the heterodimer gH/gL. Glycoprotein D (gD) acts as the receptor-binding protein, interacting with cellular receptors such as nectin-1 or HVEM. This binding triggers a conformational change that activates the gH/gL complex, which in turn triggers the fusogen gB to merge the viral and cellular membranes. The glycosylation status of these proteins is not merely decorative; it significantly influences protein folding, trafficking, and the stability of the prefusion conformations required for successful entry.
Functional Diversity of Key Glycoproteins
The functional roles of glycoproteins vary significantly across different herpesvirus subfamilies (Alpha, Beta, and Gamma). While some core machinery is conserved, the specific receptor-binding and immunomodulatory proteins differ, dictating the unique tropism and pathogenesis of each virus. The table below outlines the major glycoproteins involved in the entry and immune evasion of HSV and CMV.
| Glycoprotein | Virus Type | Primary Function | Glycosylation Feature |
|---|---|---|---|
| gB (Glycoprotein B) | HSV & CMV | Core fusion protein (fusogen) | Heavily N-glycosylated (high-mannose/hybrid); acts as a glycan shield. |
| gD (Glycoprotein D) | HSV | Receptor binding (Nectin-1, HVEM) | Contains both N- and O-linked glycans; O-glycans modulate receptor affinity. |
| gH/gL Complex | HSV & CMV | Fusion regulator; activates gB | Essential N-glycans required for proper folding and surface transport. |
| Pentameric Complex | CMV | Entry into epithelial/endothelial cells | Dense glycosylation sites on UL subunits; major target for neutralizing antibodies. |
| gE/gI Complex | HSV & VZV | Cell-to-cell spread; Fc Receptor | Bind IgG Fc region; glycosylation affects binding kinetics. |
In CMV, the complexity is further elevated. cmv glycosylation is particularly dense on the pentameric complex (gH/gL/UL128/UL130/UL131A), which is essential for infecting epithelial and endothelial cells but not fibroblasts. The varying glycosylation patterns on these subunits can alter the structural conformation of the complex, impacting its ability to bind cell surface receptors like NRP2 or PDGFRα. Furthermore, the extensive N-linked glycosylation on gB acts as a "glycan shield," protecting the fusion loops from immune recognition. Analyzing these specific glycan structures is pivotal for designing immunogens that elicit broadly neutralizing responses.
Mechanisms of Glycoprotein-Mediated Immune Evasion
Herpesviruses have evolved sophisticated strategies to utilize their surface glycoproteins not just for entry, but as active tools for immune evasion. These strategies often rely on specific glycosylation patterns or structural mimicry to deceive the host immune system. Uncovering these mechanisms provides the rationale for developing targeted therapeutic interventions.
The "Glycan Shield" Phenomenon
Viral fusion proteins, particularly HSV gB and the CMV Pentamer, effectively cloak their conserved neutralizing epitopes under a dense canopy of N-linked glycans. This steric barrier prevents neutralizing antibodies from accessing the protein core. However, this shield is rarely perfect; it often contains "holes" or distinct glycoforms that can be exploited.
Our Solution: We utilize advanced Glycosylation Analysis to map the precise topology of this shield, identifying accessible vulnerabilities that can be targeted by small-format antibodies or glycan-specific binders.
Viral Fc Receptors and Antibody Neutralization
The HSV gE/gI glycoprotein complex acts as a high-affinity viral Fc gamma receptor (vFcγR). It binds the Fc domain of host IgG antibodies, effectively "locking" them in a non-functional orientation. This prevents the recruitment of immune effector cells and blocks Antibody-Dependent Cellular Cytotoxicity (ADCC), allowing the virus to persist in the presence of an antibody response.
Our Solution: Our Anti-Herpesvirus Glycan Antibody Development platform can generate blocking antibodies that target the gE/gI interface, restoring the efficacy of the host's natural immune response.
Interference with Antigen Presentation
Certain viral glycoproteins, such as the US2-US11 series in CMV (often glycosylated type I membrane proteins), actively dislocate MHC class I molecules from the ER to the cytosol for degradation. This prevents the presentation of viral peptides to cytotoxic T cells, rendering the infected cell invisible to immune surveillance.
Our Solution: We provide specialized Custom Glycosylation services to engineer viral antigens that can bypass these evasion mechanisms, enhancing their immunogenicity for vaccine development.
Inquire About Glycoprotein Analysis
Why Partner with Creative Biolabs?
Viral Specificity
Expertise in handling complex viral envelope proteins and labile glycan structures.
Advanced Analytics
Cutting-edge mass spectrometry and SPR for kinetic and structural validation.
Rapid Development
Streamlined workflows from antigen design to antibody production and screening.
Global Support
Dedicated scientific support teams across the US, UK, and Europe to assist your project.
Published Data
A landmark study provided the first high-resolution structural insight into how a potent human antibody neutralizes Human Cytomegalovirus (HCMV) by targeting its essential fusion protein, glycoprotein B (gB). By crystallizing the viral antigenic domain II (Dom-II) in complex with the antigen-binding fragment (Fab) of the neutralizing antibody SM5-1, researchers uncovered a distinct mechanism of viral recognition. The crystal structure revealed that the antibody relies almost exclusively on two complementarity-determining regions (CDRs), utilizing an exceptionally long, rigidified CDR H3 loop to penetrate a conserved hydrophobic pocket on the surface of Dom-II.
Notably, the study demonstrated that the antibody's high affinity is driven by somatic mutations that stabilize the conformation of this long loop rather than just increasing direct antibody-antigen contacts. This structural rigidity likely enables the antibody to bind both the pre-fusion and post-fusion states of gB, thereby effectively blocking the viral fusion machinery or preventing the necessary association with the gH/gL complex. These findings define a critical vulnerability in the herpesvirus fusion apparatus and offer a structural blueprint for engineering next-generation antibodies and epitope-based vaccines with broad neutralizing potential.
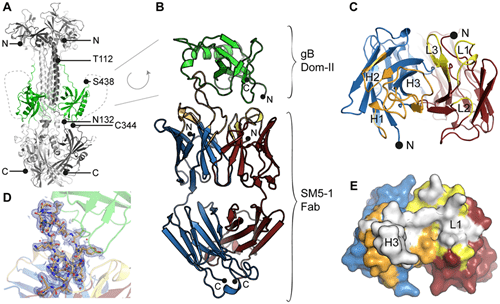 Fig.1
Crystal structure of the HCMV gB Domain II in complex with the neutralizing antibody SM5-1, illustrating the targeted epitope.1
Fig.1
Crystal structure of the HCMV gB Domain II in complex with the neutralizing antibody SM5-1, illustrating the targeted epitope.1
FAQs
How does glycosylation affect the efficacy of herpesvirus vaccines?
Glycosylation can mask conserved neutralizing epitopes ("glycan shielding") or alter the protein's conformation. Vaccines producing antigens with non-native glycosylation (e.g., expressed in bacteria or insect cells) may elicit antibodies that fail to recognize the authentic virus, leading to poor efficacy.
What is the difference between HSV-1 and HSV-2 glycoprotein structures?
While HSV-1 and HSV-2 glycoproteins (like gB and gD) share significant sequence homology (approx. 85%), they exhibit distinct glycosylation patterns and antigenic properties. These subtle differences often dictate the specific antibody response and can prevent cross-protection between the two serotypes.
Why is the CMV pentameric complex difficult to express for research?
The CMV pentamer (gH/gL/UL128/UL130/UL131A) requires the simultaneous expression and correct folding of five distinct subunits held together by disulfide bonds and hydrophobic interactions. Achieving the correct stoichiometry and native cmv glycosylation requires sophisticated mammalian expression systems.
Can you analyze O-linked glycans on herpesvirus proteins?
Yes, our Glycosylation Analysis platform includes capabilities for mapping O-linked glycans. This is particularly important for herpesviruses like HSV and VZV, where O-glycosylation on proteins like gI or gE plays a role in cell-to-cell spread and immune evasion.
Do you offer antibodies that distinguish between infected and vaccinated subjects (DIVA)?
We can develop antibodies targeting specific viral glycoproteins (like gE for VZV) that are often omitted in marker vaccines. This helps in developing DIVA assays to differentiate between natural infection and vaccine-induced immunity.
What role does the gH/gL complex play in viral entry?
The gH/gL complex acts as a regulator of the fusion process. In the hsv entry mechanism, gH/gL receives a signal from the receptor-bound gD and transmits it to the fusion protein gB, triggering the membrane fusion event necessary for viral penetration.
How do herpesviruses use glycan mimicry for immune evasion?
Herpesviruses can display host-like glycans to appear as "self" to the immune system. Additionally, proteins like the HSV gE/gI complex function as IgG Fc receptors, binding the host's own antibodies in a non-functional orientation to prevent immune effector functions like phagocytosis.
Reference:
- Spindler, Nadja, et al. "Structural basis for the recognition of human cytomegalovirus glycoprotein B by a neutralizing human antibody." PLOS Pathogens 10.10 (2014): e1004377. Distributed under Open Access license CC BY 4.0. https://doi.org/10.1371/journal.ppat.1004377
Supports
- Anti-Herpesvirus Glycan Antibody Development
- Glycoarray Platforms
- Glycosylation Analysis
- Custom Glycosylation of Biomolecules
