RSV G Protein: Structure, Glycosylation, and Immune Targets
Respiratory syncytial virus (RSV) remains a critical pathogen causing severe lower respiratory tract infections, particularly in infants and the elderly. The RSV attachment glycoprotein (G protein) is a pivotal surface antigen responsible for viral attachment and immune modulation. As a heavily glycosylated type II transmembrane protein, the RSV G protein presents unique structural challenges and opportunities for therapeutic intervention. Creative Biolabs supports cutting-edge virology research with our advanced Anti-RSV Glycan Antibody Development platform, offering precise tools to decode the complex biology of this viral glycoprotein.
CX3C Motif and Host Interaction
A defining feature of the RSV G protein is its structural homology to the human chemokine fractalkine (CX3CL1). The protein contains a conserved CX3C motif (Cys-X-X-X-Cys, specifically residues 182–186 in strain A2) located within its central conserved domain (CCD). This motif enables the G protein to function as a viral chemokine mimic, binding with high affinity to the CX3CR1 receptor expressed on airway epithelial cells and immune effector cells.
The binding of RSV G protein to CX3CR1 disrupts the host immune response by altering leukocyte chemotaxis and reducing the frequency of ciliary beating in epithelial cells, thereby impairing mucociliary clearance. Blocking this interaction is a key therapeutic strategy to reduce viral pathogenesis and airway inflammation.
Targeting the CX3C Motif
To study or block this immune-modulating interaction, high-affinity antibodies targeting the CX3C motif are essential. Creative Biolabs offers specialized Anti-RSV Glycan Antibody Development services to generate antibodies that specifically recognize this conserved domain, aiding in the development of therapeutics that preserve lung function.
Mucin-like Domains and Glycosylation
Flanking the central conserved domain are two large, hypervariable mucin-like domains. These regions are rich in serine and threonine residues, which serve as attachment sites for extensive O-linked glycosylation. Unlike N-glycans, these O-glycans are structurally diverse and can account for up to 60% of the protein’s total molecular weight.
This dense "glycan shield" creates a steric barrier that protects the viral backbone from neutralizing antibodies and proteolytic degradation. However, it also presents unique glycopeptide epitopes that can be exploited for diagnostic and therapeutic purposes. Characterizing these complex glycan structures is critical for understanding viral shielding mechanisms.
Need to Map the Glycan Shield?
We provide comprehensive Glycosylation Analysis Services using high-resolution mass spectrometry to map O-linked glycosylation sites and characterize glycan heterogeneity on viral proteins, ensuring your research data is precise and actionable.
Soluble G Protein and Immune Evasion
RSV utilizes alternative translation initiation to produce a soluble form of the G protein (sG), which lacks the transmembrane anchor. sG is secreted in large quantities into the respiratory tract, where it functions as an antigen decoy. By sharing neutralizing epitopes with the membrane-bound G protein (mG), sG effectively "soaks up" host antibodies, reducing the amount of antibody available to neutralize the infectious virus.
This decoy mechanism complicates vaccine efficacy and highlights the need for reagents that can distinguish between soluble and membrane-bound forms.
Overcoming the Decoy Effect
Differentiation between sG and mG is crucial for accurate diagnostic and mechanistic studies. Creative Biolabs offers Custom Glycosylation of Biomolecules to engineer recombinant G protein variants (secreted vs. membrane-anchored) with defined glycosylation profiles, facilitating the development of specific assays.
G Protein Variability
The G protein is the most variable gene in the RSV genome, driving the classification of RSV into groups A and B, and further into numerous genotypes. This extensive variability, driven by immune pressure on the mucin-like domains, allows the virus to evade pre-existing immunity and cause recurrent infections throughout life.
Developing broad-spectrum interventions requires identifying conserved epitopes amidst this variability or screening candidates against a wide library of viral strains.
Screening for Broad Protection
Our Glycoarray Platforms enable high-throughput screening of antibodies and lectins against diverse viral glycoforms. This powerful tool accelerates the identification of binders that recognize conserved features across multiple RSV strains.
Discuss Your RSV Project
Published Data
Effective therapeutic and diagnostic strategies against Respiratory Syncytial Virus (RSV) rely on the precise characterization of monoclonal antibodies targeting viral surface glycoproteins. A significant 2024 study provided a comprehensive analysis of a panel of 16 murine monoclonal antibodies (mAbs), rigorously evaluating their neutralizing potential and epitope specificity against diverse viral strains. Utilizing high-throughput competitive ELISA and virus-neutralization assays, researchers successfully mapped nine distinct antigenic target sites on the viral fusion protein. The structural analysis revealed a complex spatial organization wherein five sites partially overlapped to form a major antigenic determinant, while four others—comprising three neutralizing and one non-neutralizing site—remained structurally distinct. Crucially, the investigation demonstrated the strict conformational dependence of these interactions; the highly potent antibodies bound effectively to native oligomeric forms (dimers and trimers) but exhibited significantly reduced reactivity with denatured monomeric structures. Furthermore, the selection of escape mutants under antibody pressure identified specific amino acid residues, such as N240, that are critical for maintaining the integrity of neutralizing epitopes. These findings underscore the absolute necessity of utilizing conformationally native antigens and advanced epitope mapping techniques during the discovery phase to ensure the isolation of potent, broadly reactive binders capable of recognizing the antigenic diversity of contemporary circulating RSV strains.
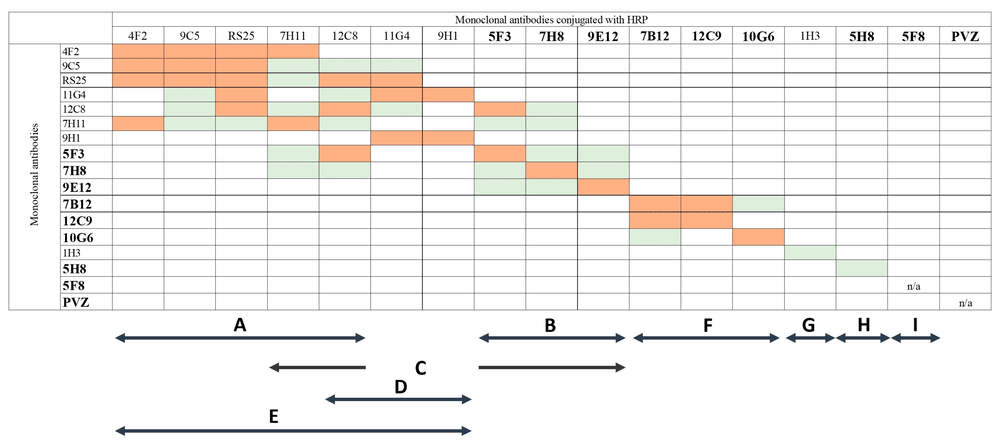 Fig.1
Competitive epitope mapping of RSV-specific monoclonal antibodies using inhibition ELISA.1
Fig.1
Competitive epitope mapping of RSV-specific monoclonal antibodies using inhibition ELISA.1
FAQs
Why is the RSV G protein considered a difficult target for antibody development?
The primary challenge is the dense shield of O-linked glycans in the mucin-like domains, which can sterically hinder antibody binding. Additionally, the G protein is highly variable between strains, and the secretion of a soluble decoy form (sG) can absorb neutralizing antibodies before they reach the virus.
What is the significance of the central conserved domain (CCD)?
The CCD is nearly identical across RSV group A and B strains. It contains the CX3C motif required for binding to the host receptor CX3CR1. Antibodies targeting this region are often broadly neutralizing and can block viral attachment, making it a prime vaccine target.
Does glycosylation affect the immunogenicity of the G protein?
Yes, significantly. Glycosylation can mask protein epitopes (decreasing immunogenicity) but can also create unique glycopeptide epitopes. The specific pattern of glycosylation, determined by the host cell, influences how the immune system perceives the antigen.
Can you develop antibodies that distinguish between RSV Group A and Group B?
Yes. By targeting the hypervariable mucin-like domains rather than the conserved central domain, we can generate strain-specific antibodies useful for diagnostic typing and epidemiological surveillance.
Do you offer services to analyze the O-glycans on the RSV G protein?
Absolutely. Our Glycosylation Analysis platform uses mass spectrometry to characterize the site occupancy and structure of O-linked glycans, providing a detailed map of the viral glyco-shield.
Reference:
- Krivitskaya, V., et al. "Characterization of a Panel of Monoclonal Antibodies Targeting the F-Protein of the Respiratory Syncytial Virus (RSV) for the Typing of Contemporary Circulating Strains." Tropical Medicine and Infectious Disease 9.1 (2024): 1. Distributed under Open Access license CC BY 4.0. https://doi.org/10.3390/tropicalmed9010001
Supports
- Glycoarray Platforms Overview
- Glycosylation Analysis Services
- Custom Glycosylation of Biomolecules
- Anti-RSV Glycan Antibody Development
