Decoding the "Sulfation Code" of Glycosaminoglycans
Glycosaminoglycans (GAGs) are not mere structural fillers in the extracellular matrix; they are sophisticated information carriers. The biological information of GAGs, such as heparan sulfate (HS) and chondroitin sulfate (CS), is encoded primarily in their sulfation patterns. This "sulfation code" dictates how GAGs interact with hundreds of signaling proteins, including growth factors, chemokines, and morphogens, thereby regulating critical processes like embryonic development, angiogenesis, and tumor metastasis. At Creative Biolabs, we are pioneers in deciphering these complex carbohydrate languages. Through our Anti-GAG Sulfation Motif Neo-Epitope Antibody Development service, we provide researchers with precise tools to visualize and manipulate specific sulfation motifs, moving beyond generic GAG detection to functional dissection.
What is the "Sulfation Code"?
The "sulfation code" hypothesis posits that specific sequences of sulfated sugars within GAG chains act as high-affinity binding sites for regulatory proteins. Unlike DNA or proteins, which are synthesized based on a template, GAGs are synthesized in the Golgi apparatus through a non-template-driven process involving a complex interplay of glycosyltransferases and sulfotransferases. This results in significant structural heterogeneity, or "micro-heterogeneity," where the information is dense and spatially regulated.
Structural Vocabulary of Major GAGs
The diversity of the GAG sulfation pattern arises from the combinatorial modification of disaccharide units. The following table summarizes the key sulfation positions that constitute the "code" for major GAG classes.
| GAG Type | Disaccharide Unit | Key Modification Sites (The Code) |
|---|---|---|
| Heparan Sulfate (HS) | GlcNAc/GlcNS - GlcA/IdoA |
|
| Chondroitin Sulfate (CS) | GalNAc - GlcA |
|
| Dermatan Sulfate (DS) | GalNAc - IdoA |
|
Biological Mechanisms & Pathology
How the Code Regulates Function: The GAG-Protein Interactome
Specific sulfation motifs act as "landing pads" for proteins. For instance, the interaction between Fibroblast Growth Factor 2 (FGF2) and its receptor (FGFR) requires a ternary complex with heparan sulfate. This interaction is not promiscuous; it strictly demands a specific sulfation motif containing N-sulfate and 2-O-sulfate groups. Similarly, chemokine oligomerization on endothelial surfaces—a prerequisite for leukocyte recruitment during inflammation—is driven by specific GAG sequences. These interactions demonstrate that the sulfation code acts as a molecular switch, turning signaling pathways on or off depending on the presence of the correct sugar motif.
Developmental Signaling
During embryogenesis, gradients of morphogens (like Wnt, Hedgehog, and BMP) are shaped by extracellular GAGs. Specific sulfation patterns limit the diffusion of these factors, creating precise concentration gradients that define tissue boundaries and cell fate. Loss of specific sulfotransferases often leads to severe developmental defects.
Cancer & Metastasis
Tumor cells often hijack the GAG synthesis machinery. They may alter their surface sulfation code to promote growth factor signaling or shed GAGs to remodel the extracellular matrix. Furthermore, the enzyme heparanase cleaves HS chains, releasing sequestered growth factors and facilitating tumor invasion.
Applications
Deciphering the GAG sulfation code is not just an academic exercise; it has profound implications for translational research and therapy. By using high-affinity neo-epitope antibodies, researchers can now interrogate biological systems with unprecedented resolution.
Cancer Research
Identify tumor-specific sulfation signatures for biomarker discovery and target metastatic niche formation.
Neuroscience
Map perineuronal nets (PNNs) and study plasticity by targeting specific chondroitin sulfate motifs.
Infectious Disease
Block viral entry (e.g., HSV, SARS-CoV-2) by targeting the GAG co-receptors essential for infection.
Developmental Biology
Investigate stem cell fate decisions regulated by GAG-mediated morphogen gradients.
Our Solutions: Targeting the Code with Neo-Epitope Antibodies
Studying the sulfation code has historically been hampered by a lack of specific tools. Traditional lectins and chemical stains lack the resolution to distinguish between subtle isomer differences (e.g., GlcNAc-6S vs. GlcNAc-6OH). To bridge this gap, Creative Biolabs offers a specialized platform for generating neo-epitope antibodies that recognize distinct GAG sulfation motifs.
Custom Neo-Epitope Antibody Generation
We utilize phage display technology to screen for single-chain variable fragments (scFv) or VHHs that bind exclusively to defined sulfated oligosaccharides. By using synthetic, chemically defined GAG fragments as targets, we eliminate the ambiguity of natural heterogeneous samples.
Glycoarray Specificity Profiling
Validation is key. We screen candidate antibodies against our high-density GAG glycoarray, which contains hundreds of distinct sulfation variants. This ensures that your antibody binds the intended motif (e.g., HS-3S) without cross-reacting with closely related structures (e.g., HS-6S).
Sequencing & Structural Analysis
For clients needing to characterize the sulfation code of their biological samples, we offer advanced LC-MS/MS GAG sequencing. We can quantify the abundance of specific disaccharides in your tissue or cell line, correlating structural data with antibody staining results.
Custom GAG Synthesis
Need a specific GAG standard? We provide enzymatic or chemical synthesis of oligosaccharides with defined lengths and sulfation patterns. These serve as perfect controls for inhibition assays or structural studies.
Workflow: From Design to Delivery
Inquire about Neo-Epitope Antibodies
Published Data
Glycosaminoglycans (GAGs) have evolved from mere structural scaffolds into sophisticated instructive biomolecules that actively regulate cellular behavior and synaptic function. Recent open-access data elucidates how the "sulfation code"—specific patterns of sulfation on Heparan Sulfate (HS) and Chondroitin Sulfate (CS) chains—orchestrates the assembly of perineuronal nets (PNNs) in the central nervous system. As visualized in the accompanying figure, these specialized matrix structures form tight, lattice-like networks around neurons, providing essential stabilization for synaptic junctions. The study establishes that these interactions are not random; they are driven by distinct sulfation motifs that act as high-affinity binding sites for neuroregulatory proteins such as tenascin-R and semaphorins. This specific molecular architecture creates a buffered microenvironment that preserves membrane polarization and optimizes neurotransmission efficiency. Crucially, the data demonstrates that the functional state of neural networks is dependent on the spatiotemporal expression of these specific sulfated epitopes. Consequently, deciphering these complex regulatory mechanisms requires high-precision tools, such as neo-epitope antibodies, to selectively target and visualize the bioactive motifs responsible for synaptic plasticity and tissue homeostasis, distinguishing them from the constitutive background of the extracellular matrix.
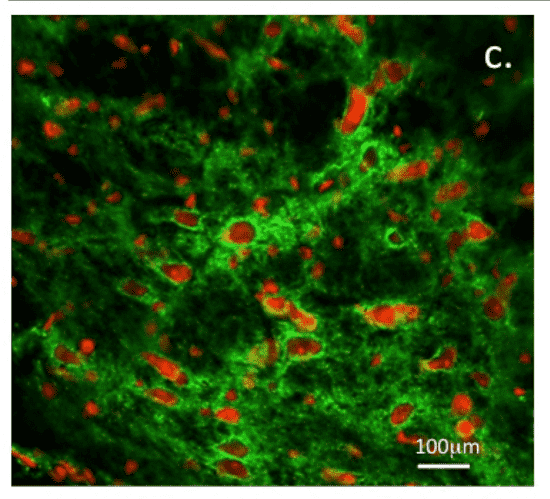 Fig.1 Structural organization of perineuronal nets mediated by specific GAG sulfation motifs.1
Fig.1 Structural organization of perineuronal nets mediated by specific GAG sulfation motifs.1
FAQs
Why are "neo-epitope" antibodies better than traditional GAG antibodies?
Traditional antibodies (like IgM class markers) often have broad specificity and low affinity. "Neo-epitope" antibodies are designed against short, chemically defined sequences. This allows them to distinguish between subtle structural differences, such as 6-O-sulfation versus 2-O-sulfation, providing "sequencing-grade" resolution for your experiments.
Can you generate antibodies against rare sulfation motifs like 3-O-sulfated Heparan Sulfate?
Yes. 3-O-sulfation is rare but biologically potent (e.g., for HSV entry). Because we use synthetic antigens, we can enrich for binders against these rare structures, even if they are low-abundance in natural tissues.
Do these antibodies work in immunohistochemistry (IHC)?
Yes, many of our phage-display derived antibodies (especially when converted to scFv-Fc or IgG formats) work well in IHC and IF. However, due to the fixation sensitivity of carbohydrates, we recommend specific protocols which we provide upon delivery.
How do you ensure the antibody doesn't cross-react with DNA or other negatively charged polymers?
We perform counter-selection steps during the phage display panning process. We include excess DNA, hyaluronic acid, and over-sulfated chondroitin sulfate in the blocking buffer to deplete non-specific charge-based binders, ensuring the final clones recognize the specific stereochemistry of the sugar, not just the negative charge.
What format are the final antibodies delivered in?
We offer flexible formats. The initial binders are usually selected as scFv or VHH. These can be delivered as periplasmic extracts or purified proteins. For stability and ease of use, we highly recommend engineering them into full-length IgG (rabbit, mouse, or human backbone) or scFv-Fc fusion proteins.
Reference:
- Melrose, J. "Glycosaminoglycans, Instructive Biomolecules That Regulate Cellular Activity and Synaptic Neuronal Control of Specific Tissue Functional Properties." International Journal of Molecular Sciences 26.6 (2025): 2554. Distributed under Open Access license CC BY 4.0. https://doi.org/10.3390/ijms26062554
