Chondroitin Sulfate Proteoglycans (CSPGs) in Neural Regeneration
The failure of the adult central nervous system (CNS) to regenerate after injury is a major challenge in neuroscience and medicine. Among the complex factors contributing to this failure, the extracellular matrix (ECM) plays a pivotal role. Specifically, chondroitin sulfate proteoglycans (CSPGs) are upregulated significantly following trauma, forming a chemical barrier known as the glial scar. While this scar serves an acute protective role, it ultimately prevents axonal regrowth and plasticity. At Creative Biolabs, we provide comprehensive Anti-Chondroitin Sulfate (CS) Antibody Development services to facilitate research into these inhibitory molecules. Our goal is to equip researchers with high-specificity tools to dissect the roles of various sulfation patterns and develop therapeutic strategies for cspg spinal cord injury models and beyond.
Understanding CSPGs: Structure and Function
Chondroitin sulfate proteoglycans (CSPGs) constitute a diverse class of extracellular matrix molecules essential for CNS homeostasis. The primary families expressed in the nervous system include the lecticans—comprising aggrecan, brevican, neurocan, and versican—as well as phosphacan and the transmembrane proteoglycan NG2. Structurally, lecticans are defined by a core protein featuring globular N-terminal (G1) and C-terminal (G3) domains, which facilitate interactions with hyaluronan and tenascins, respectively. These core proteins are substituted with chondroitin sulfate glycosaminoglycan (CS-GAG) chains, the sulfation patterns of which dictate biological activity. In the healthy adult CNS, CSPGs condense to form Perineuronal Nets (PNNs) around specific neuronal somata and dendrites. These lattice-like structures stabilize synaptic connections and restrict plasticity, maintaining the mature neural circuit architecture.
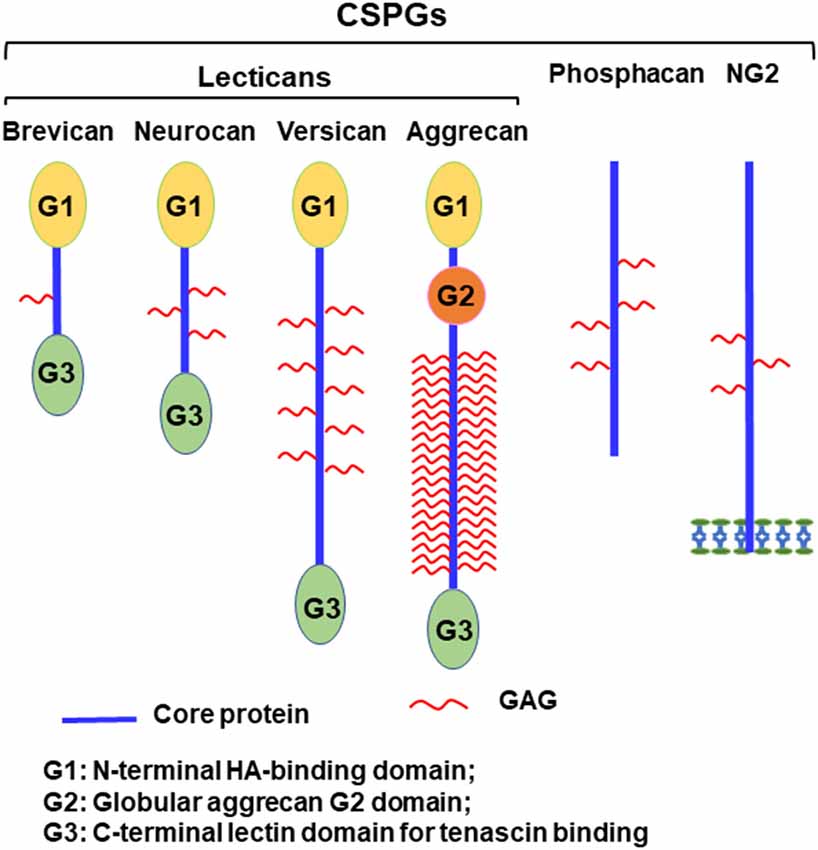 Fig.1 Structural diversity of major CNS Chondroitin Sulfate Proteoglycans (CSPGs), detailing the core protein domains (G1, G2, G3) and glycosaminoglycan (GAG) attachment sites.1
Fig.1 Structural diversity of major CNS Chondroitin Sulfate Proteoglycans (CSPGs), detailing the core protein domains (G1, G2, G3) and glycosaminoglycan (GAG) attachment sites.1
The Glial Scar and Neural Regeneration Inhibition
In the context of traumatic injury or neurodegenerative disease, the beneficial role of CSPGs shifts toward the inhibition of repair. Reactive astrocytes and other glial cells at the lesion site rapidly upregulate CSPG expression, contributing to the formation of the glial scar. This scar tissue acts as a formidable chemical barrier to regeneration. CSPGs exert their inhibitory effects primarily through their sulfated GAG chains, which sterically hinder the binding of axonal integrins to growth-promoting substrates like laminin. Furthermore, CSPGs potentiate the effects of chemorepulsive molecules, such as Semaphorin 3A and 5A, and limit calcium entry into neurons. Crucially, this inhibition is mediated by high-affinity interactions between GAG chains and specific neuronal transmembrane receptors, including Protein Tyrosine Phosphatase sigma (PTPσ), Leukocyte Common Antigen-Related (LAR) phosphatase, and Nogo Receptors (NgR1 and NgR3). Activation of these receptors triggers intracellular signaling cascades, notably the RhoA/ROCK pathway, leading to growth cone collapse and the cessation of axon elongation.
Mechanisms of Inhibition: Receptors and Signaling
The inhibitory effects of CSPGs are not merely physical; they are mediated by specific signaling pathways triggered when GAG chains bind to receptors on the neuronal surface.
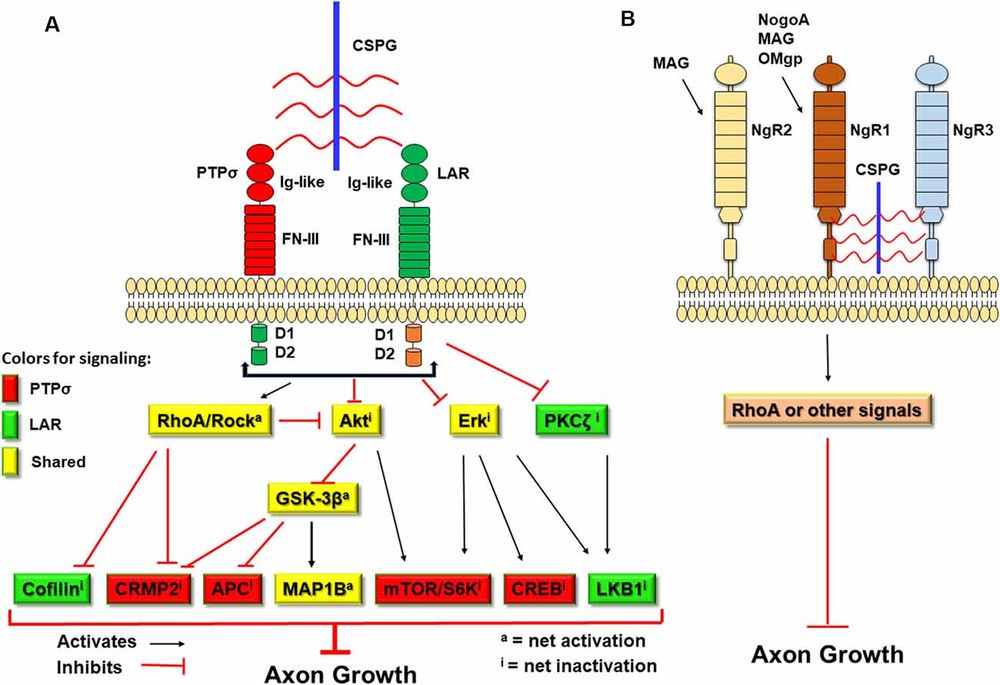 Fig.2 Transmembrane receptors PTPσ and LAR mediate CSPG-induced growth inhibition through convergent intracellular signaling involving RhoA activation and Akt/Erk inactivation.1
Fig.2 Transmembrane receptors PTPσ and LAR mediate CSPG-induced growth inhibition through convergent intracellular signaling involving RhoA activation and Akt/Erk inactivation.1
Protein Tyrosine Phosphatase Sigma (PTPσ)
PTPσ is a transmembrane receptor that acts as a primary sensor for CSPGs. The binding of CS chains to PTPσ triggers downstream signaling cascades, including the Rho/ROCK pathway, which leads to growth cone collapse and the cessation of axon extension. Blocking PTPσ or its interaction with CSPGs has been shown to promote regeneration.
Leukocyte Common Antigen-Related (LAR) Phosphatase
Similar to PTPσ, the LAR receptor binds to CSPGs and transduces inhibitory signals. It has been implicated in restricting axonal growth in various CNS injury models. Interventions that target the LAR receptor have demonstrated potential in enhancing synaptic plasticity and recovery.
Nogo Receptors (NgR1 and NgR3)
While classically associated with myelin-associated inhibitors, Nogo receptors (particularly NgR1 and NgR3) have also been identified as high-affinity binding partners for CSPGs. This cross-talk suggests a convergent pathway for multiple inhibitory cues within the glial scar, reinforcing the blockade against regeneration.
Therapeutic Strategies and Our Services
Given the critical role of CSPGs in preventing recovery, therapeutic strategies focus on neutralizing their inhibitory activity. Two main approaches are currently under investigation:
- Enzymatic Digestion: Bacterial enzyme Chondroitinase ABC (ChABC) digests GAG chains, removing the inhibitory sugar structures. While effective in animal models, its instability and immunogenicity pose clinical challenges.
- Immunotherapy: Using antibodies to block specific inhibitory motifs on CS chains or to block the receptors (like PTPσ). This offers a more targeted approach with potentially fewer side effects.
Creative Biolabs supports this frontier of research with specialized antibody development services.
Anti-Chondroitin Sulfate (CS) Antibody Development
We provide a complete platform for generating antibodies against specific CS motifs. Whether you are targeting the 4-sulfated (CS-A), 6-sulfated (CS-C), or highly inhibitory CS-E epitopes, our team can design immunogens and screen libraries (phage display or hybridoma) to identify high-affinity binders. These antibodies are essential tools for:
- Mapping the Glial Scar: visualizing the spatial distribution of specific sulfation patterns in tissue sections.
- Functional Blocking: Neutralizing the inhibitory activity of CSPGs in in vitro neurite outgrowth assays or in vivo injury models.
- Drug Delivery: Targeting nanoparticles or enzymes specifically to the lesion site using anti-CS antibodies as homing moieties.
Inquire About Anti-CSPG Antibody Projects
Published Data
The failure of the central nervous system (CNS) to regenerate following trauma or neurodegenerative disease is largely attributed to the rapid upregulation of Chondroitin Sulfate Proteoglycans (CSPGs) at the lesion site. A comprehensive review highlights that while the expression of CSPGs—including the lectican family (aggrecan, versican, neurocan, brevican), phosphacan, and NG2—initially serves a protective role by containing tissue damage, these molecules ultimately coalesce to form a potent chemical barrier known as the glial scar. This extracellular matrix becomes highly inhibitory to repair, primarily driven by the glycosaminoglycan (GAG) side chains attached to the protein core. Specific sulfation patterns, such as CS-A and CS-E, interact with receptors like PTPσ and LAR on neuronal growth cones, triggering intracellular signaling cascades (Rho/ROCK) that result in dystrophic endbulbs and the cessation of axonal extension. Furthermore, these proteoglycans severely restrict the migration and differentiation of oligodendrocyte precursor cells (OPCs), thereby impeding remyelination efforts. Therapeutic strategies that neutralize these inhibitory signals, such as the enzymatic digestion of GAG chains via Chondroitinase ABC or the targeting of specific sulfation motifs, have demonstrated significant potential in reopening a window for neuroplasticity and promoting axonal sprouting in various models of spinal cord injury.
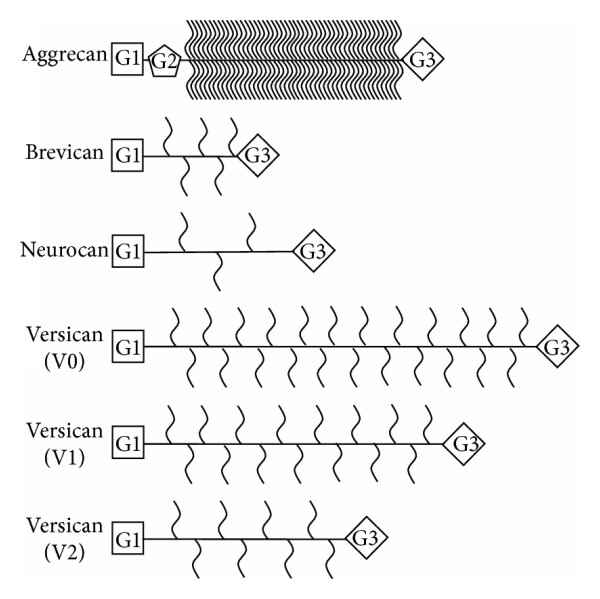 Fig.3 Structural diversity of Chondroitin Sulfate Proteoglycans (CSPGs) in the CNS, highlighting the core proteins and inhibitory glycosaminoglycan (GAG) side chains.2
Fig.3 Structural diversity of Chondroitin Sulfate Proteoglycans (CSPGs) in the CNS, highlighting the core proteins and inhibitory glycosaminoglycan (GAG) side chains.2
FAQs
What makes CSPGs inhibitory to nerve regeneration?
The inhibition is primarily driven by the sulfated glycosaminoglycan (GAG) side chains attached to the protein core. Specific sulfation patterns, such as 4-sulfation (CS-A) and 4,6-disulfation (CS-E), bind to receptors like PTPσ and LAR on neurons, triggering intracellular pathways (e.g., Rho/ROCK) that stop axon growth.
How does the glial scar affect spinal cord injury recovery?
The glial scar acts as a physical and chemical barrier. While it contains inflammation initially, the dense meshwork of CSPGs produced by reactive astrocytes within the scar prevents axons from regenerating across the injury site, leading to permanent functional deficits in cspg spinal cord injury cases.
Can antibodies be used to treat CSPG-mediated inhibition?
Yes, research suggests that antibodies targeting specific inhibitory CS motifs or their receptors can mask these sites, preventing the inhibitory signal transduction. This approach is being explored as a more targeted alternative to broad enzymatic digestion with Chondroitinase ABC.
What is the difference between CS-A, CS-C, and CS-E?
These refer to different sulfation patterns on the chondroitin sulfate disaccharide units. CS-A is sulfated at position 4, CS-C at position 6, and CS-E is disulfated at positions 4 and 6. CS-E and CS-A are generally considered more inhibitory to axonal growth than CS-C.
Does Creative Biolabs provide antibodies for detecting specific GAG patterns?
Yes, we offer custom development of anti-glycan antibodies that can distinguish between subtle structural differences, including specific sulfation patterns on CSPGs, using our advanced phage display and glycan array screening platforms.
References:
- Sami, Armin, Michael E. Selzer, and Shuxin Li. "Advances in the signaling pathways downstream of glial-scar axon growth inhibitors." Frontiers in Cellular Neuroscience 14 (2020): 174. Distributed under Open Access license CC BY 4.0. https://doi.org/10.3389/fncel.2020.00174
- Siebert, Justin R., Amanda Conta Steencken, and Donna J. Osterhout. "Chondroitin sulfate proteoglycans in the nervous system: inhibitors to repair." BioMed research international 2014.1 (2014): 845323. Distributed under Open Access license CC BY 3.0. https://doi.org/10.1155/2014/845323
