Genetic Modification of the Host Biosynthesis Pathway
Different glycoforms of antibodies significantly influence their effector functions, including ADCC and CDC. Therefore, it's essential to control antibody glycosylation in host cell lines and modulate the associated biosynthesis pathways to achieve desired glycoforms. Leveraging advanced knowledge in glycobiology, Creative Biolabs has become a sought-after partner for manufacturing specific antibodies with customized effector functions. We have developed a range of sophisticated genetic strategies for glycoengineering host cells according to customer specifications, aiming to elucidate the mechanisms of glycan actions in various biological processes.
Genetic Modifications of the Host Biosynthesis Pathway at Creative Biolabs
Creative Biolabs offers a comprehensive suite of genetic engineering services to optimize host cell biosynthesis pathways. Our expertise lies in precisely modifying host cells to produce antibodies with specific glycosylation patterns. Our service involves various aspects encompassing glyco-site engineering, pathway optimization, glycosyltransferase expression, and metabolic engineering. By manipulating key enzymes and regulatory factors involved in host biosynthetic pathways, we are able to fine-tune the production of antibodies with enhanced effector functions. During the whole process, our experienced experts develop exclusive solutions to cater to the various needs of our customers. Leveraging our powerful platform, we are committed to providing researchers around the world with high-quality solutions with excellent performance to facilitate the discovery and development of efficient therapeutics.
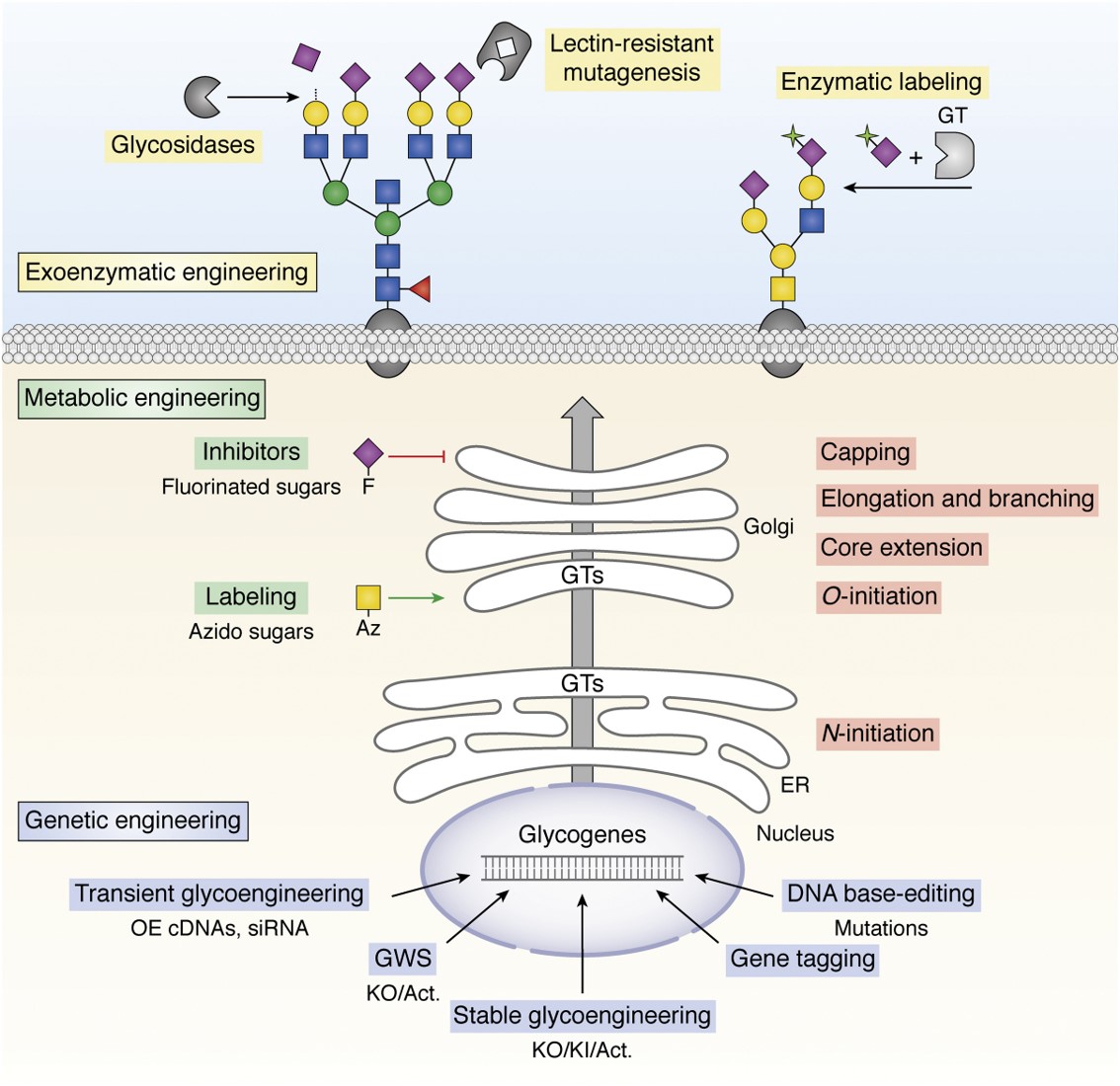 Fig.1 An integration of glycoengineering strategies.1
Fig.1 An integration of glycoengineering strategies.1
Multiple Genetic Modifications Strategies in Host Glycosylation
Glycosylation is a crucial post-translational modification of recombinant antibodies that significantly influences their biological activity, immunogenicity, in vivo metabolism, and both antibody-dependent and complement-dependent cytotoxicity. Here, we provide several genetic modification strategies to control the glycosylation in host cells, including:
- Knockout of specific glycosyltransferases: Knockout of genes encoding glycosyltransferases that catalyze the addition of specific sugar residues using gene editing can prevent the formation of unwanted glycan structures and can redirect metabolic flux to alternative pathways to produce different glycan structures.
- Overexpression of glycosyltransferases: Overexpression of specific glycosyltransferases can promote the addition of specific sugar residues, resulting in the accumulation of desired glycan structures. Overexpression of certain glycosyltransferases can alter the overall glycosylation pathway, resulting in new glycan structures.
- Introduction of novel glycosyltransferases: Introducing genes encoding glycosyltransferases from other organisms using gene editing can expand the library of glycan structures that can be produced in host cells, and combining endogenous and exogenous glycosyltransferases can produce mixed glycan structures with unique properties.
- Metabolic engineering: Manipulation of metabolic pathways can increase the availability of specific sugar precursors, affect the glycosylation process, and regulate the activity of metabolic enzymes.
- Anti-Apoptosis: By introducing anti-apoptosis genes, the regulation and autophagy in host cells can be inhibited to prolong cell life and improve the survival rate of host cells.
Highlight Features
 Fig.2 Our service advantages.
Fig.2 Our service advantages.
Frequently Asked Question
Q1: How can the safety of genetically modified antibodies be ensured?
A1: We conduct thorough testing and stringent quality assurance protocols to guarantee the safety of genetically engineered antibodies. This comprehensive process starts with the detailed characterization of the modified cells and proteins. Our scientists meticulously analyze the genetic modifications, protein expression levels, and post-translational modifications to assess potential impacts on the antibody's structure and function. Subsequently, toxicity testing in animal models is conducted to evaluate the safety profile of the genetically modified antibody. These trials aid in identifying potential side effects and assessing the antibody's tolerance. We also provide clinical support to aid customers in achieving meaningful projects. These trials involve carefully designed studies that monitor patient responses, side effects, and therapeutic outcomes. By combining these rigorous testing and quality control measures, we enable global customers to develop safe and effective genetically modified antibody therapies.
If you are looking for a reliable and competent partner to work together, please feel free to get in touch with us with your meaningful projects.
Reference
- Narimatsu, Yoshiki, et al. "Genetic glycoengineering in mammalian cells." Journal of Biological Chemistry 296 (2021). Distributed under Open Access License CC BY 4.0, without modification.
For Research Use Only.
