Bile Duct Ligation (BDL) induced Primary Biliary Cirrhosis Modeling & Pharmacodynamics Service
Creative Biolabs provides a wide range of animal models for evaluating liver fibrosis and cirrhosis. These models replicate the pathophysiological features of human liver diseases, offering an ideal platform for testing novel therapies. With accurate and reproducible results, our services support drug development and the optimization of therapeutic strategies for liver fibrosis and cirrhosis.
Introduction
Liver fibrosis is a condition in which the liver undergoes progressive scarring due to chronic injury, commonly caused by viral infections (hepatitis B and C), alcohol abuse, metabolic disorders, and autoimmune diseases. In response to prolonged damage, hepatocytes (liver cells) are replaced by fibrous tissue, disrupting normal liver architecture. As fibrosis advances, it can lead to cirrhosis, a late-stage liver disease marked by widespread scarring, the formation of regenerative nodules, and liver dysfunction. Cirrhosis impairs vital liver functions such as bile production, nutrient processing, and detoxification, often leading to complications like ascites, hepatic encephalopathy, variceal bleeding, and liver failure. Cirrhosis is also a precursor to hepatocellular carcinoma (HCC), a leading cause of liver cancer worldwide. The early stages of liver fibrosis are often asymptomatic, making early detection challenging. Therefore, advancements in diagnostic tools and therapeutic strategies are crucial to halting the progression of fibrosis before it evolves into cirrhosis. Current research aims at identifying biomarkers for early diagnosis and developing treatments that can reverse or prevent liver fibrosis from progressing to cirrhosis.
Disease Models and Applications
The Bile Duct Ligation (BDL) induced Primary Biliary Cirrhosis Model is a widely used animal model for studying cholestatic liver diseases, particularly primary biliary cirrhosis. In this model, the bile duct is surgically ligated, leading to bile accumulation in the liver, which results in hepatic fibrosis, inflammation, and cirrhosis. This model mimics human cholestatic liver diseases, providing insight into the pathophysiology of biliary obstruction and cirrhosis. One of its key advantages is the development of progressive fibrosis, which can be quantified over time. However, a limitation of the model is its surgical invasiveness, which may introduce variability and affect reproducibility. Nevertheless, it remains a cornerstone for testing therapies aimed at biliary fibrosis and cirrhosis.
- Simulates: The Bile Duct Ligation (BDL) induced Primary Biliary Cirrhosis Model simulates cholestatic liver diseases, such as primary biliary cirrhosis (PBC) and secondary biliary cirrhosis, which are characterized by bile duct obstruction, liver fibrosis, and inflammation.
- Evaluates Drugs: This model is used to evaluate drugs targeting biliary fibrosis, liver regeneration, anti-inflammatory therapies, and cholestasis. It is particularly useful for screening compounds aimed at reducing liver fibrosis, preventing liver failure, and improving liver function in cholestatic liver diseases.
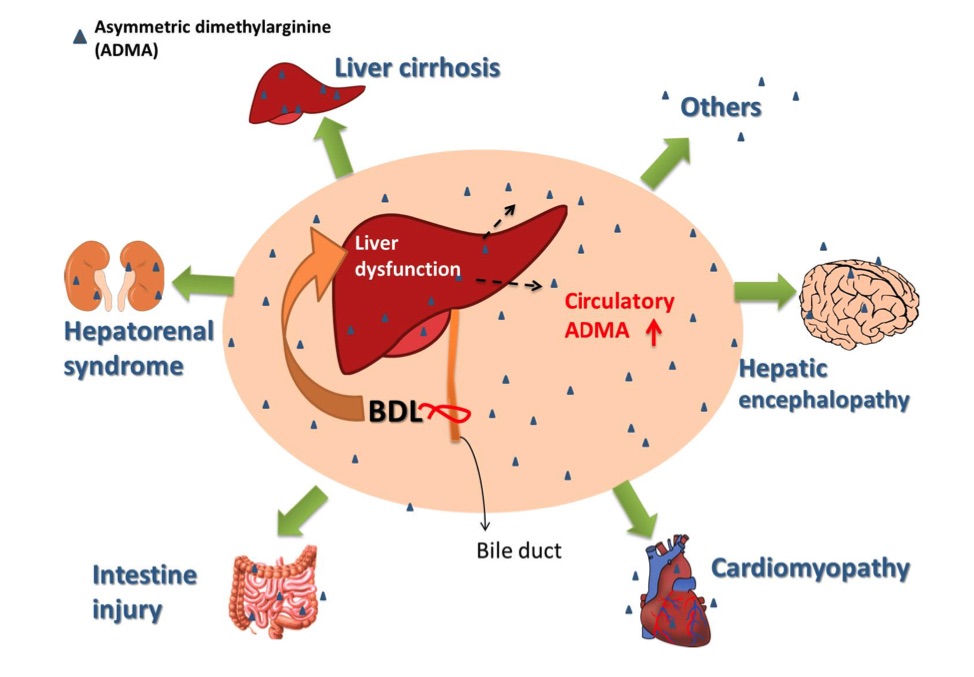 Fig. 1 The role of increased circulatory asymmetric dimethylarginine (ADMA) in multiple organ damage in the bile duct ligation rat.1
Fig. 1 The role of increased circulatory asymmetric dimethylarginine (ADMA) in multiple organ damage in the bile duct ligation rat.1
Measurements
We offer a range of measurements for evaluating drug efficacy in the Bile Duct Ligation (BDL) induced Primary Biliary Cirrhosis Model, utilizing cutting-edge technologies, such as:
- General Observations: Body weight, liver size, liver enzymes, and bilirubin levels.
- Histopathology: Liver tissue examination via H&E staining for fibrosis, inflammation, and biliary obstruction.
- Immunohistochemistry: Detection of immune cell infiltration (e.g., macrophages, T-cells) and bile ductular proliferation in liver tissue.
- Serum Biomarkers: Assessment of liver function via serum ALT, AST, GGT, and alkaline phosphatase levels.
- Fibrosis Staging: Quantification of hepatic fibrosis using methods like Masson's trichrome staining or Sirius Red staining for collagen deposition.
- Gene/Protein Profiling: RT-qPCR and Western blot analysis to assess the expression of fibrosis markers such as collagen, α-SMA, and fibronectin.
Additionally, our team can customize experiments based on specific needs, ensuring that each model addresses research questions, including drug efficacy and mechanistic insights into cholestatic liver disease.
Related Services
In addition to the Bile Duct Ligation (BDL) induced Primary Biliary Cirrhosis Model, we offer alternative models of liver injury, including those induced by carbon tetrachloride (CCl4), acetaminophen (APAP), and other toxicants. These models allow for a broader evaluation of drug effects across different etiologies of liver damage, providing comprehensive insights into drug efficacy. We also provide tailored support for researchers to select the most appropriate model, assisting in experimental design and data analysis.
- CCL4 induced Liver Fibrosis/Cirrhosis Model
- Thioacetamide (TAA) induced Liver Fibrosis/Cirrhosis Model
- Alpha-Naphthylisothiocyanate (ANIT) induced Primary Biliary Cirrhosis Model
- 3,5-Diethoxycarbonyl-1,4-Dihydrocollidine (DDC) induced Primary Biliary Cirrhosis Model
Advantages
- Advanced Technology: Our state-of-the-art technologies enable precise monitoring of disease progression and therapeutic effects, offering superior data quality.
- Comprehensive Expertise: With years of experience in liver disease modeling, our team provides expert guidance throughout the entire research process—from model selection to data analysis.
- Reproducibility and Consistency: We ensure high reproducibility in all our models, guaranteeing reliable and consistent results for your drug evaluation studies.
- Robust Data Support: Our comprehensive data analysis services help you gain deeper insights from experimental results, supporting more informed decision-making.
- Fast Turnaround: We prioritize efficient project management to ensure timely delivery of results, helping you stay on track with your research timelines.
- Ethical and Regulatory Compliance: Our models adhere to the highest ethical standards and regulatory guidelines, ensuring the validity and integrity of your studies.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What types of Acute Liver Injury models do you offer?
We provide various ALI models, including chemical induced models (e.g., acetaminophen, CCl4), viral hepatitis models, and ischemic injury models. These models are designed to simulate different mechanisms of liver damage for a comprehensive assessment of drug efficacy.
-
2. How long does it take to see results from the Acute Liver Injury models?
The timeline depends on the specific model and the severity of the injury induced. Typically, initial liver damage and therapeutic effects can be observed within 1–2 weeks, with longer-term effects, such as recovery or progression to fibrosis, taking up to 4–6 weeks.
-
3. Can the models be used for chronic liver disease studies?
Yes, some of our ALI models progress to chronic conditions like liver fibrosis and cirrhosis, allowing you to study both acute and chronic stages of liver disease, including treatment strategies for long-term damage.
-
4. Are the models suitable for testing hepatotoxic drugs?
Absolutely. Our models are ideal for evaluating hepatotoxicity and studying the mechanisms behind liver injury induced by various chemical compounds, enabling preclinical screening of potential drug candidates.
-
5. Do you offer support for experimental design and data analysis?
Yes, we offer comprehensive support, including consultation on experimental design, model selection, and post-experiment data analysis. Our scientific team ensures you receive the insights you need for your research.
Published Data
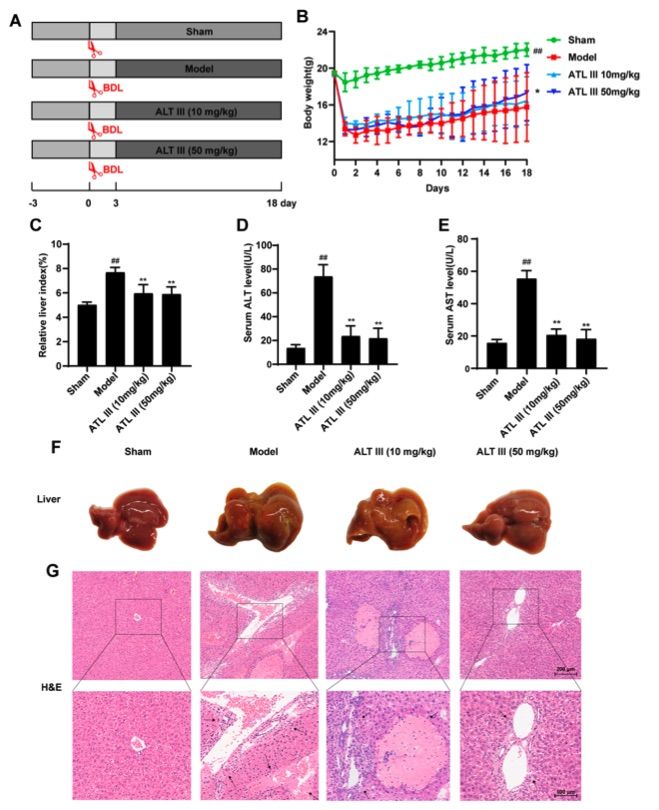 Fig. 2 ATL III inhibited BDL induced liver injury.2
Fig. 2 ATL III inhibited BDL induced liver injury.2
The effect of ATL III on liver injury induced by bile duct ligation (BDL) was evaluated at two doses, 10 mg/kg and 50 mg/kg. Both doses significantly reduced the extent of liver damage. To assess the impact on BDL induced liver fibrosis, ATL III-treated groups were compared with the sham and model groups. BDL surgery caused a substantial decrease in the body weight of mice compared to the Sham group, with recovery observed after ATL III administration. This recovery was statistically significant when compared to the model group (Figure 2A, B). Liver index measurements showed a significant increase in the model group, while both ATL III treatment groups demonstrated a significant decrease in liver index compared to the sham group (Figure 2C). Serum ALT and AST levels were notably reduced following ATL III treatment (Figure 2D, E). Morphological examination revealed that the liver surface of the model group was rough with yellow spots, in contrast to the smoother liver surface with fewer yellow spots observed in the ATL III-treated groups (Figure 2F). Histological analysis revealed extensive necrosis, severe liver structural damage, and destruction of the lobular architecture in the model group, along with hepatic ballooning, collagen deposition, and bile duct hyperplasia. In contrast, these pathological features were significantly diminished in both ATL III-treated groups (Figure 2G). These findings suggest that ATL III administration alleviates liver injury caused by BDL and may have therapeutic potential in liver fibrosis.
References
- Sheen, Jiunn-Ming et al. "Increased circulatory asymmetric dimethylarginine and multiple organ failure: bile duct ligation in rat as a model." International journal of molecular sciences vol. 15,3 3989-4006. 5 Mar. 2014, DOI:10.3390/ijms15033989. Distributed under an Open Access license CC BY 4.0, without modification.
- Wang, Yan et al. "Atractylenolide III Ameliorates Bile Duct Ligation induced Liver Fibrosis by Inhibiting the PI3K/AKT Pathway and Regulating Glutamine Metabolism." Molecules (Basel, Switzerland) vol. 28,14 5504. 19 Jul. 2023, DOI:10.3390/molecules28145504. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
