Concanavalin A (Con A) induced Acute Liver Injury Modeling & Pharmacodynamics Service
Creative Biolabs offers a range of well-established animal models for acute liver injury to support drug discovery, providing robust services for assessing therapeutic candidates.
Introduction
Acute liver injury (ALI) is a serious condition characterized by rapid liver damage and dysfunction, often triggered by toxins, infections, or immune reactions. Common causes of ALI include viral hepatitis, alcohol abuse, drug toxicity, and ischemic injury. The liver’s regenerative ability allows for recovery in mild cases, but severe injury can lead to liver failure, requiring a transplant. Acute liver injury is marked by elevated liver enzymes, jaundice, coagulopathy, and hepatocellular necrosis. Early diagnosis and intervention are crucial in preventing the progression of ALI to chronic liver diseases such as cirrhosis. Animal models of ALI are widely used in preclinical studies to investigate mechanisms of liver injury and test potential therapies. These models play a crucial role in identifying novel treatments for liver diseases, providing insight into liver pathology, and evaluating drug efficacy.
Disease Models and Applications
The Concanavalin A (Con A) induced Acute Liver Injury Model is one of the most commonly used models for studying immune-mediated liver injury. Con A, a plant lectin, is injected into rodents to induce a robust immune response, leading to hepatocyte injury, inflammation, and liver dysfunction. This model primarily mimics acute T-cell-mediated liver damage, providing insight into the immune mechanisms behind liver injuries seen in autoimmune diseases, viral hepatitis, and drug induced liver injury. Con A induced liver injury is characterized by liver inflammation, elevated liver enzymes, hepatocyte apoptosis, and fibrosis. The key advantage of this model is its ability to replicate the immune mechanisms underlying ALI, particularly T-cell-mediated hepatic inflammation. However, one limitation is that the model does not fully mimic the broad spectrum of injuries seen in human ALI, as it primarily reflects immune-driven liver damage. Despite this, the Con A model remains a valuable tool for investigating the role of immune cells in liver injury and testing immunomodulatory drugs.
- Simulates: The Concanavalin A (Con A) induced Acute Liver Injury Model simulates immune-mediated liver damage, particularly in the context of T-cell activation and inflammation. This model is useful for studying the pathogenesis of autoimmune liver diseases, viral hepatitis, and drug induced liver toxicity.
- Evaluates Drugs: This model is widely used for evaluating drugs targeting immune modulation, inflammation, and hepatocyte protection. Specifically, it is suitable for testing anti-inflammatory agents, immunosuppressive drugs, and agents that target liver cell apoptosis, such as corticosteroids or immunotherapies.
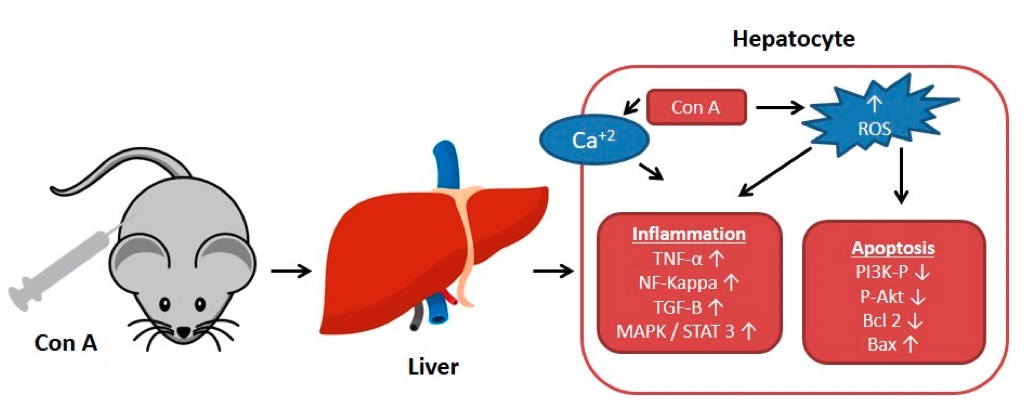 Fig. 1 Effect of concanavalin A (Con A) on the molecular level in Con A induced hepatotoxicity.1
Fig. 1 Effect of concanavalin A (Con A) on the molecular level in Con A induced hepatotoxicity.1
Measurements
We offer a variety of measurements for evaluating drug efficacy in rodent acute liver injury models, utilizing an array of advanced technologies, including but not limited to:
- General Observations: Body weight, mortality rate, stool consistency, and gastrointestinal bleeding.
- Histopathology: Liver tissue analysis via H&E staining to assess necrosis, inflammation, and hepatocyte damage.
- Immunohistochemistry: Infiltration of immune cells (e.g., T-cells, macrophages) in liver tissues.
- Cytokine Profiling (e.g., ELISA): Expression levels of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β.
- Liver Enzyme Levels: Serum biomarkers like ALT, AST, and alkaline phosphatase to assess liver damage.
- Gene/Protein Expression Profiling: RT-qPCR and Western blot techniques to measure key molecular markers of liver injury, fibrosis, and inflammation.
In addition to established models of acute liver injury, our expertise extends to developing custom models tailored to specific research needs, guided by literature and prior studies. Our scientific team is available to assist in experimental design, model selection, and data analysis, ensuring an effective and customized approach for each project.
Related Services
In addition to the Concanavalin A (Con A) induced Acute Liver Injury Model, we also offer other models. These models provide alternative methods for studying various aspects of acute liver injury and allow for the testing of a broader range of therapeutic interventions.
- CCL4 induced Acute Liver Injury Model
- Polyinosinic: Polycytidylic Acid induced Acute Liver Injury Model
- Acetaminophen (APAP) induced Acute Liver Injury Model
- Alcohol induced Acute Liver Injury Model
- Ischemia-Reperfusion induced Liver Injury Model
- Alpha-Naphthylisothiocyanate (ANIT) induced Acute Liver Injury Model
- DDC (3,5-Diethoxycarbonyl-1,4-Dihydrocollidine) induced Acute Liver Injury Model
Advantages
- Tailored Solutions: We offer flexible and customized models to suit your specific research needs.
- Advanced Technology: Our models utilize the latest technologies for accurate and reliable assessment of drug efficacy.
- Experienced Scientific Team: Our team offers expert guidance from experimental design to data analysis.
- Comprehensive Support: We provide end-to-end services, from model development to drug evaluation and biomarker analysis.
- Rapid Turnaround: We ensure timely results to accelerate the progress of your research and development.
- High Reproducibility: Our models are validated for consistent and reproducible results across experiments.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What is the primary use of the Concanavalin A (Con A) induced Acute Liver Injury Model?
This model is primarily used to study immune-mediated liver injury, such as in autoimmune hepatitis or viral liver diseases. It helps understand the role of T-cells and inflammatory cytokines in liver damage.
-
2. How does the Con A model induce liver injury?
Concanavalin A (Con A) induces liver injury through activation of T-cells and subsequent liver inflammation, which leads to hepatocyte apoptosis and tissue damage.
-
3. What types of drugs can be evaluated using the Con A model?
Drugs that modulate immune responses, reduce inflammation, or protect hepatocytes from apoptosis can be evaluated in this model, including corticosteroids, immunosuppressants, and anti-inflammatory agents.
-
4. What histological features are examined in this model?
Key histological features include hepatocyte necrosis, infiltration of immune cells, and liver inflammation, typically assessed using H&E staining and immunohistochemistry.
-
5. How long does it take to establish the Concanavalin A (Con A) induced Acute Liver Injury Model?
It typically takes 24 to 48 hours to induce significant liver injury after a single injection of Con A, with peak liver damage observed within 24 hours post-injection.
Published Data
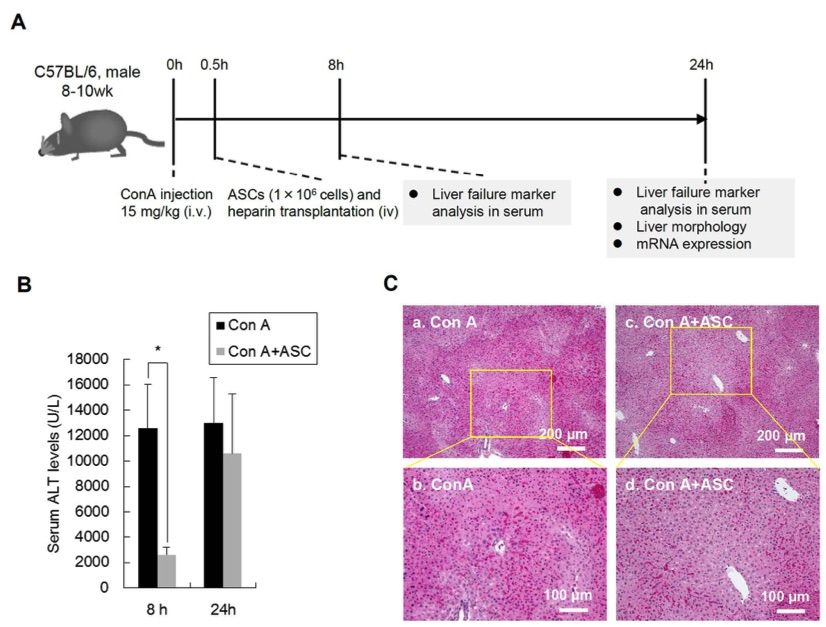 Fig. 2 Effect of ASC transplantation on Con A induced liver injury in mice.2
Fig. 2 Effect of ASC transplantation on Con A induced liver injury in mice.2
To assess the effects of transplanted adipose-derived stem cells (ASCs) on liver injury induced by Concanavalin A (ConA), serum ALT levels were measured at 8 and 24 hours post-ConA administration (Fig. 2A). At 8 hours, the ALT levels in the ConA group were significantly elevated (12,617 ± 3,431 U/L). In contrast, the ASC-transplanted group (ConA+ASCs) exhibited a marked decrease in ALT levels at the same time point (2,633 ± 578 U/L). At 24 hours post-ConA administration, ALT levels in the ConA+ASCs group were elevated (10,617 ± 4,684 U/L) compared to the 8-hour time point but remained lower than the levels in the ConA group at 24 hours (13,017 ± 3,563 U/L) (Fig. 2B). Histological examination of liver tissue at 24 hours revealed extensive necrotic areas in the ConA group, characterized by acidophilic cytoplasm and condensed nuclei in hepatocytes. In contrast, the necrotic areas in the ConA+ASCs group were smaller and less severe (Fig. 2C). These findings, including the changes in ALT levels and histopathological observations, suggest that ASC transplantation may offer therapeutic benefits in treating fulminant hepatic failure (FHF) induced by excessive immune responses.
References
- Ibrahim, Sabrin R M et al. "Summary of Natural Products Ameliorate Concanavalin A induced Liver Injury: Structures, Sources, Pharmacological Effects, and Mechanisms of Action." Plants (Basel, Switzerland) vol. 10,2 228. 25 Jan. 2021, DOI:10.3390/plants10020228. Distributed under an Open Access license CC BY 4.0, without modification.
- Yoshizumi, Yasuma et al. "Immunomodulatory Effects of Adipose Tissue-Derived Stem Cells on Concanavalin A induced Acute Liver Injury in Mice." Cell medicine vol. 9,1-2 21-33. 6 Oct. 2016, DOI:10.3727/215517916X693159. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
