Gout Modeling & Pharmacodynamics Services
Creative Biolabs provides a variety of well-established rodent models for evaluating drug efficacy in gout. These models simulate key aspects of human gout, including uric acid overproduction and crystal induced inflammation. We offer comprehensive assessments, including joint inflammation, serum uric acid levels, and histological examination, to support preclinical testing of potential gout therapies. Our team can guide you through model selection, experimental design, and data analysis to ensure effective and accurate results.
Introduction
Gout is a form of inflammatory arthritis characterized by sudden and severe attacks of pain, redness, and swelling, primarily affecting the joints. It occurs due to the accumulation of uric acid crystals in the joints, resulting in inflammation. High levels of uric acid in the blood (hyperuricemia) are the primary cause, which may be due to overproduction of uric acid or underexcretion by the kidneys. The most common site of attack is the big toe, but it can also affect other joints like the ankles, knees, and elbows. There are two main types of gout: acute gout, which involves sudden flare-ups of pain and swelling, and chronic gout, characterized by frequent flare-ups and long-term joint damage. Gout can also lead to the formation of tophi, which are lumps of urate crystals that can develop under the skin around the affected joints.
Disease Models and Applications
Creative Biolabs offers a comprehensive range of well-established rodent models for gout, including models for acute and chronic gout induced by uric acid overproduction or impaired renal excretion. These models are carefully designed to replicate key features of human gout, such as the formation of uric acid crystals in joints, inflammatory responses, and hyperuricemia. Along with the models, we provide extensive evaluations of various parameters, including joint inflammation, serum uric acid levels, histological changes, and cytokine profiling. This enables accurate preclinical assessment of potential therapeutic candidates for gout. Our experienced team of scientists will work closely with you throughout your project, from experimental design to data interpretation, ensuring high-quality and reliable results. To learn more about the rodent gout models available for preclinical research, please explore the links below:
| Model | Simulated Disease | Drug Evaluation Focus | Animal species |
| Monosodium Urate (MSU) induced Gout Model | Gout, acute arthritis, uric acid deposition | Anti-inflammatory drugs, urate-lowering agents, pain relief therapies, immune modulators, inhibitors of uric acid crystallization | Rat |
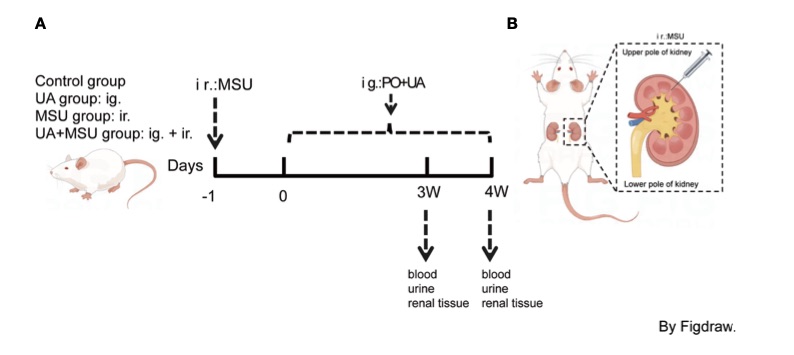 Fig. 1 Flowchart of strategic study design in animal model (rat).1
Fig. 1 Flowchart of strategic study design in animal model (rat).1
Measurements
We offer a variety of measurements for evaluating drug efficacy in rodent gout models, utilizing an array of advanced technologies, including but not limited to:
General observations: Body weight, mortality rate, joint swelling, limping, and signs of discomfort associated with acute or chronic gout flare-ups.
Histopathology: Examination of joint tissues using H&E and polarized light microscopy to detect urate crystal deposition, joint inflammation, and cartilage damage.
Cytokine profiling (e.g., ELISA): Measurement of inflammatory mediators such as IL-1β, TNF-α, IL-6, and C-reactive protein (CRP), which are involved in the inflammatory response in gout.
Serum biomarkers: Monitoring of serum uric acid levels, kidney function markers (e.g., creatinine, BUN), and liver enzymes (e.g., ALT, AST) to assess metabolic changes and therapeutic effects.
Gene/protein expression profiling via RT qPCR and Western blot: Analysis of key genes and proteins involved in gout pathogenesis, such as NLRP3 inflammasome, IL-1β, and collagen type I, to evaluate the molecular response to treatment.
In addition to the established gout models, our expertise extends to the development of novel animal models tailored to specific research needs, guided by the latest literature and prior studies. Our scientific team is available to assist in experimental design, model selection, and data analysis, ensuring a customized and effective approach to your project at every stage.
Related Services
In addition to gout models, we also offer a wide range of models for other diseases. These models enable comprehensive evaluation across diverse therapeutic areas.
Advantages
- Expertise in Preclinical Research: With years of experience in developing and optimizing animal models, our team is highly skilled in delivering accurate and reliable preclinical results. We specialize in gout research and offer a deep understanding of disease mechanisms, ensuring that your studies are in capable hands.
- Comprehensive and Customized Solutions: We provide tailored animal models that are specifically designed to meet your research objectives. Whether you are studying acute gout, chronic gout, or urate crystal deposition, we can adapt models to suit your needs. Our solutions are flexible and highly customizable, ensuring relevant and precise results.
- Advanced Technologies and Analytical Capabilities: Our models are supported by cutting-edge technologies for detailed assessments, such as histopathology, cytokine profiling, serum biomarkers, and gene/protein expression analysis. We offer state-of-the-art methods like RT-qPCR, Western blotting, and polarized light microscopy for comprehensive evaluation of therapeutic efficacy.
- Validated Models for Gout Research: We use well-established, validated rodent models of gout, ensuring that you receive consistent and reproducible data that closely mimics human disease conditions. These models are ideal for evaluating drug efficacy, safety, and mechanism of action in gout research.
- Full Support from Design to Data Interpretation: Our team works closely with you throughout your research journey. From experimental design and model selection to data analysis and interpretation, we provide the guidance and support necessary to achieve high-quality results.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What types of gout models do you offer?
We offer well-established rodent models for both acute and chronic gout, including models induced by monosodium urate (MSU) crystals and high uric acid levels. These models closely mimic human gout, including joint inflammation, urate crystal deposition, and hyperuricemia.
-
2. Can the models be customized for specific research needs?
Yes, our gout models are fully customizable. Whether you need to investigate acute gout flare-ups, chronic gout, or the effects of novel therapies, we can tailor the models to your specific research objectives and therapeutic candidates.
-
3. What measurements are included in the evaluation of gout models?
We offer comprehensive evaluations, including general observations (e.g., body weight, joint swelling), histopathology (urate crystal deposition, joint damage), serum biomarker analysis (uric acid levels, kidney function), and cytokine profiling (IL-1β, TNF-α, IL-6). We also provide gene/protein expression analysis using techniques such as RT-qPCR and Western blotting.
-
4. How do you assess the severity of gout in these models?
The severity of gout is assessed through histopathological evaluation of joint tissues (looking for urate crystals, inflammation, and cartilage damage), serum biomarker measurements (such as uric acid levels), and histological scoring systems. These parameters are used to calculate a histological severity score (HSS) for each treatment group.
-
5. What drugs or therapies can be tested in these models?
Our gout models are ideal for testing a wide range of therapeutics, including uric acid-lowering agents like allopurinol, nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, novel biologic therapies targeting IL-1β, and drugs aimed at enhancing renal excretion of uric acid.
-
6. How long does it take to obtain results from your models?
The timeline depends on the complexity of the study and the model used. Typically, preliminary results can be obtained within a few weeks, with comprehensive data available after thorough histological analysis and data interpretation.
Published Data
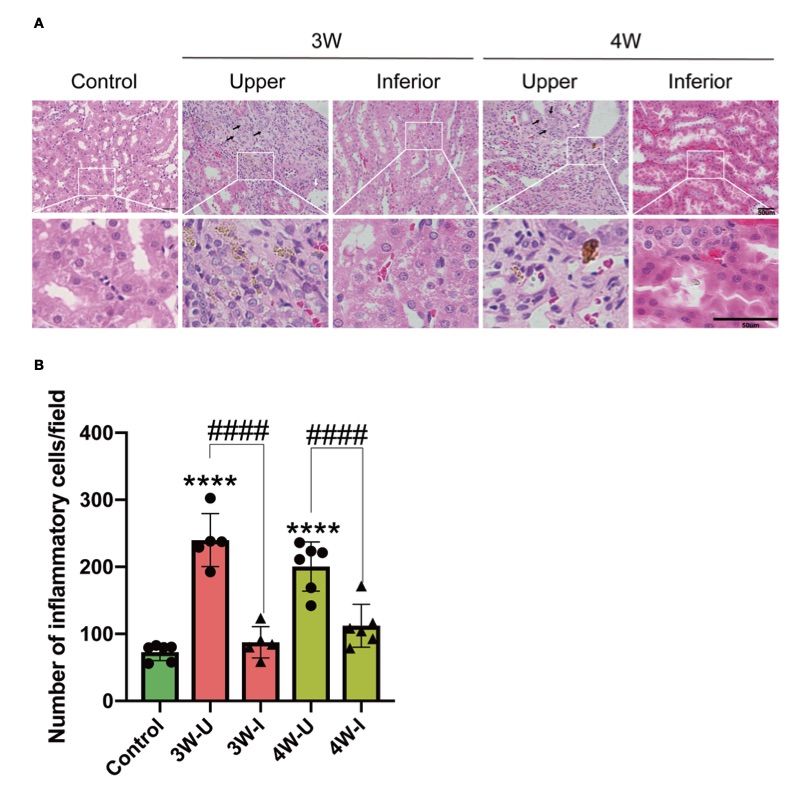 Fig. 2 Intrarenal MSU injection caused localized renal tubular damage and an increase in inflammatory cell infiltration.1
Fig. 2 Intrarenal MSU injection caused localized renal tubular damage and an increase in inflammatory cell infiltration.1
This article introduced a novel approach involving the intrarenal injection of monosodium urate (MSU) crystals to induce glomerulonephritis (GN) in rats. The goal was to investigate the pathological effects of MSU crystals on kidney tissue, providing a basis for future research on the mechanisms and prevention of GN. Intrarenal administration of MSU crystals resulted in considerable damage to the peripheral renal tubules, characterized by tubular dilatation, vacuolar degeneration, and significant infiltration of inflammatory cells into the renal interstitium. (A) Hematoxylin and eosin (H&E) staining of kidney sections from the experimental rats (n = 6; mean ± SEM) showed distinct damage in the upper pole (injected with MSU crystals) compared to the lower pole (injected with PBS). The scale bar represents 50 µm. Arrows in the images highlight the damage: the white arrow indicates tubular dilatation, while the black arrow points to vacuolar degeneration. (B) The infiltration of inflammatory cells into the renal interstitium was quantified at three and four weeks (3W/4W) post-injection. The data show significant inflammatory cell accumulation in the upper pole (3W-U/4W-U) compared to the lower pole (3W-I/4W-I) of the kidney, highlighting the pathological effects of MSU crystals on renal tissue.
Reference
- Li, Delun et al. "The pathogenic mechanism of monosodium urate crystal induced kidney injury in a rat model." Frontiers in endocrinology vol. 15 1416996. 1 Jul. 2024, doi:10.3389/fendo.2024.1416996. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
