Ischemia-Reperfusion induced Liver Injury Modeling & Pharmacodynamics Service
Creative Biolabs provides a range of well-established models, including the Ischemia-Reperfusion induced Liver Injury Model, to evaluate potential therapeutic agents targeting ALI. These models are optimized to provide reliable, reproducible, and accurate results for drug evaluation and mechanistic studies.
Introduction
Acute Liver Injury (ALI) is a rapid and severe loss of liver function that can occur due to various insults, including drug toxicity, infections, and ischemia. It is marked by hepatocyte damage, inflammation, oxidative stress, and coagulation abnormalities. If untreated, ALI can lead to multi-organ failure, liver cirrhosis, and even death. The ischemia-reperfusion (IR) injury model is an experimental tool used to mimic liver damage caused by restricted blood flow followed by reperfusion, which is commonly observed in conditions like liver surgery, transplantation, or shock. In this model, liver injury is induced by temporarily occluding the blood supply to the liver, followed by the restoration of blood flow. This process results in cellular damage, inflammation, and oxidative stress, like what is seen in clinical scenarios of ischemic liver injury.
Disease Models and Applications
The Ischemia-Reperfusion induced Liver Injury (IR-ILI) model is constructed by temporarily clamping the hepatic artery or portal vein in rodents, followed by reperfusion, which simulates the conditions of ischemic liver injury that occur during surgeries such as liver transplantation or liver resection. The model is typically used to assess the liver’s response to re-establishment of blood flow and evaluate the mechanisms of liver damage, including inflammation, oxidative stress, and cell death. This model closely mimics human clinical conditions, such as ischemia-reperfusion injury in liver transplantation, making it a valuable tool for testing potential therapeutic agents. However, the model also has some limitations, such as the variability in response based on animal strain and difficulty in controlling the exact timing of reperfusion. Nevertheless, the IR-ILI model remains a crucial experimental approach for studying liver injury and recovery after ischemia.
- Simulates: The Ischemia-Reperfusion induced Liver Injury model simulates the liver damage caused by ischemia and the subsequent restoration of blood flow, which is common in liver transplantation, resection, and trauma.
- Evaluates Drugs: This model is used to evaluate drugs that aim to reduce liver injury, protect hepatocytes, or modulate inflammation and oxidative stress. It is particularly useful for testing hepatoprotective agents, antioxidants, anti-inflammatory compounds, and drugs that enhance liver regeneration after ischemic damage.
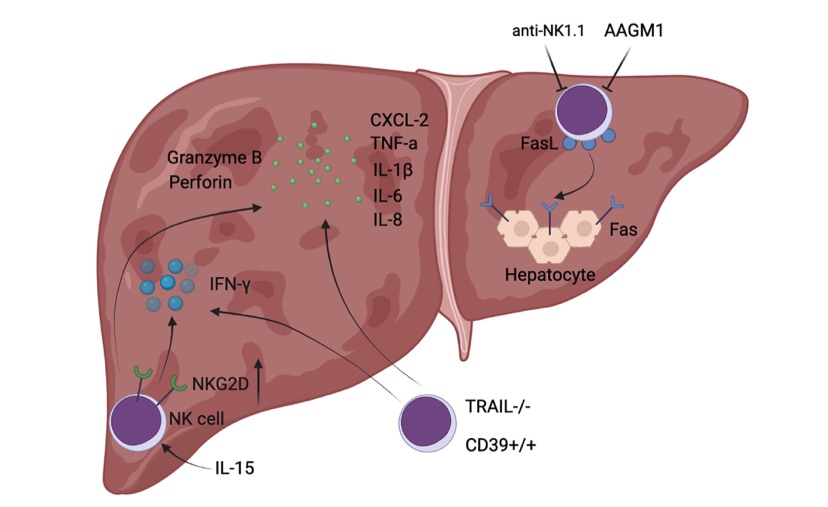 Fig. 1 Diagrammatic sketch of NK cell-mediated exacerbated liver injury during hepatic I/R.1
Fig. 1 Diagrammatic sketch of NK cell-mediated exacerbated liver injury during hepatic I/R.1
Measurements
To assess the efficacy of drugs in the Alcohol induced Acute Liver Injury Model, a variety of advanced measurements are employed, including:
- General observations: body weight, mortality rate, liver appearance, and general health of the animal.
- Histological analysis: Hematoxylin and eosin (H&E) staining to observe liver tissue damage, hepatocyte apoptosis, and inflammatory cell infiltration.
- Cytokine profiling (e.g., ELISA): Levels of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β.
- Liver enzyme assays: Serum levels of liver enzymes (AST, ALT) as biomarkers of liver injury.
- Oxidative stress markers: Malondialdehyde (MDA) and glutathione (GSH) levels to measure oxidative damage.
- Gene/protein expression: RT-qPCR and Western blotting to evaluate changes in the expression of key liver enzymes and stress-related genes.
In addition to these standard assessments, our team is available to customize experimental protocols based on your specific research needs.
Related Services
In addition to the Ischemia-Reperfusion induced Liver Injury Model, we offer several other methods to induce Acute Liver Injury. These models include drug induced liver injury, diet induced models, and toxin-based models, which can be tailored to specific research purposes. We ensure that each model is optimized for reliable and reproducible results, providing a robust foundation for drug discovery and mechanistic studies.
- CCL4 induced Acute Liver Injury Model
- Concanavalin A (Con A) induced Acute Liver Injury Model
- Polyinosine-Polycytidylic Acid induced Acute Liver Injury Model
- Acetaminophen (APAP) induced Acute Liver Injury Model
- Alcohol induced Acute Liver Injury Model
- Alpha-Naphthylisothiocyanate (ANIT) induced Acute Liver Injury Model
- DDC (3,5-Diethoxycarbonyl-1,4-Dihydrocollidine) induced Acute Liver Injury Model
Advantages
- Customized models: We offer a wide range of flexible and customizable animal models, allowing for specific research needs.
- Expertise: Our team consists of experts in drug development and animal model optimization to guide every phase of your research.
- Advanced technology: We utilize state-of-the-art technologies for data collection and analysis, ensuring the most accurate and reliable results.
- Comprehensive services: From experimental design to post-study analysis, we provide a full range of services to ensure your research is successful.
- High reproducibility: Our models are carefully optimized to provide consistent, reproducible results across multiple studies.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What is the duration of the Alcohol induced Acute Liver Injury model?
The duration can vary depending on the specifics of your experiment, but typically, the model involves a 24–72-hour observation period after ethanol administration.
-
2. How do you standardize ethanol doses in the model?
Dosing is calibrated based on literature and animal strain to ensure consistent and reproducible results without causing chronic liver damage.
-
3. Can the AI-ALI model be used to assess non-alcoholic therapies?
Yes, the model can be adapted to test a range of therapeutic agents, including those targeting inflammation, oxidative stress, and liver regeneration pathways.
-
4. Is the model suitable for long-term drug studies?
While primarily designed for acute injury, we can modify the protocol to allow for chronic studies by adjusting dosing regimens and treatment durations.
Published Data
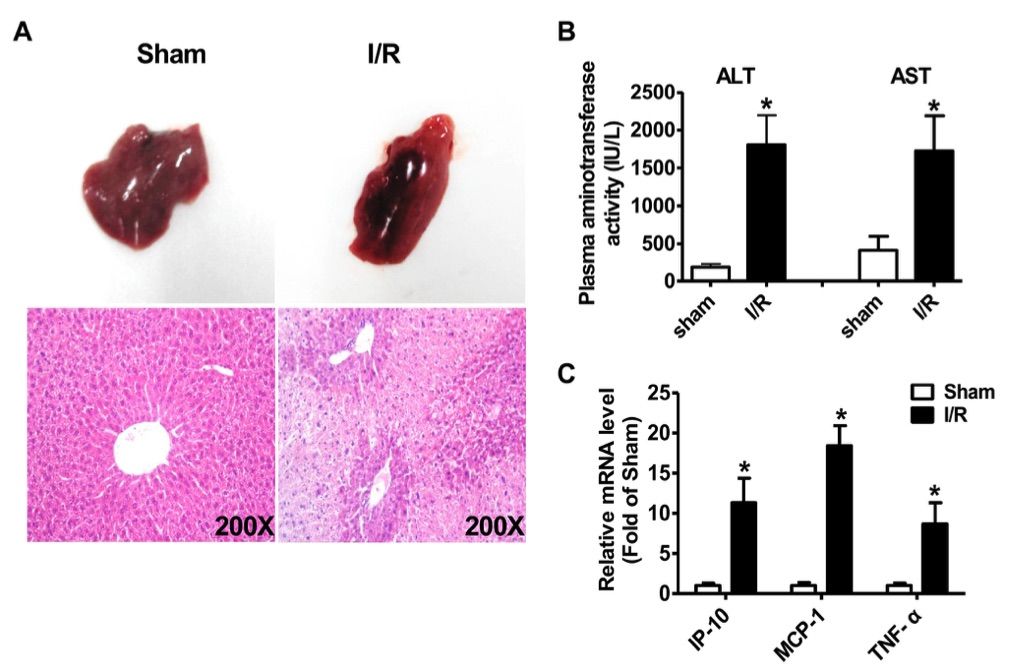 Fig. 2 Characterics of liver injury after ischemia/reperfusion treatment.2
Fig. 2 Characterics of liver injury after ischemia/reperfusion treatment.2
To investigate the potential role of long non-coding RNAs (LncRNAs) in hepatic ischemia/reperfusion injury, a partial hepatic ischemia/reperfusion model was established in mice. The protocol involved inducing 70% partial hepatic ischemia for 1 hour, followed by reperfusion for 6 hours at room temperature. After the reperfusion period, the mice were euthanized, and liver and plasma samples were collected for analysis. Morphological examination and H&E staining revealed significant liver injury (Figure 2A), accompanied by markedly elevated plasma levels of AST and ALT (Figure 2B). Additionally, the expression of inflammatory cytokines, including IP-10, MCP-1, and TNF-α, was significantly increased in the ischemia/reperfusion-injured livers compared to sham-treated controls (Figure 2C). These pathophysiological changes confirmed the successful establishment of the hepatic ischemia/reperfusion injury model in this study.
References
- Huang, Miao et al. "Natural Killer Cells in Hepatic Ischemia-Reperfusion Injury." Frontiers in immunology vol. 13 870038. 28 Mar. 2022, DOI:10.3389/fimmu.2022.870038. Distributed under an Open Access license CC BY 4.0, without modification.
- Chen, Zhenzhen et al. "Silencing of long noncoding RNA AK139328 attenuates ischemia/reperfusion injury in mouse livers." PloS one vol. 8,11 e80817. 27 Nov. 2013, DOI: 10.1371/journal.pone.0080817. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
