Alpha-Naphthylisothiocyanate (ANIT) induced Primary Biliary Cirrhosis Modeling & Pharmacodynamics Service
Creative Biolabs offers a range of well-established models to evaluate the efficacy of drugs targeting Primary Biliary Cirrhosis (PBC). These models, including both animal and cell-based systems, are designed to mimic the pathophysiology of PBC, enabling comprehensive testing of therapeutic candidates.
Introduction
Primary Biliary Cirrhosis (PBC) is a chronic autoimmune liver disease characterized by progressive destruction of the small bile ducts within the liver. This leads to bile buildup, which causes liver damage, inflammation, and ultimately cirrhosis. The condition is more common in women and typically presents between the ages of 30 and 60. The exact cause of PBC is unknown, but it is believed to involve genetic and environmental factors, such as infections or chemicals, that trigger an immune response against the bile ducts. Symptoms include fatigue, pruritus (itching), jaundice, and right upper quadrant pain. In the later stages, PBC can progress to liver failure, requiring a liver transplant for survival. PBC is often diagnosed through a combination of blood tests showing elevated liver enzymes, positive anti-mitochondrial antibodies (AMA), and a liver biopsy to assess the degree of liver damage. Although there is no cure for PBC, treatment focuses on managing symptoms and slowing the disease progression. Ursodeoxycholic acid (UDCA) is commonly used as a first-line therapy to improve bile flow and liver function. Immunosuppressive treatments, such as corticosteroids and novel biologics, are also under investigation.
Disease Models and Applications
The Alpha-Naphthylisothiocyanate (ANIT) induced Primary Biliary Cirrhosis Model is widely used to study cholestatic liver diseases. The model is established by administering ANIT to rodents, typically rats, which induces biliary injury and mimics human primary biliary cirrhosis (PBC). The model closely resembles the pathological characteristics of PBC, such as bile duct damage, inflammation, and fibrosis. It is an excellent tool for testing potential therapies targeting bile flow impairment and liver fibrosis. While this model provides valuable insight into the progression of biliary cirrhosis, its limitations include the fact that ANIT induced toxicity does not fully replicate the autoimmune aspect of human PBC. Despite this, it remains a powerful tool for preclinical drug evaluation.
- Simulates: The ANIT induced model simulates cholestatic liver injury and is used to study primary biliary cirrhosis (PBC) in humans, including bile duct damage and fibrosis.
- Evaluates Drugs: The model is ideal for evaluating potential therapeutic agents, particularly those aimed at restoring bile flow, reducing inflammation, and mitigating fibrosis in cholestatic liver diseases. Drugs that modulate immune responses or fibrosis, as well as those targeting liver regeneration, can be effectively tested using this model.
Measurements
We offer a comprehensive range of measurements to assess the efficacy of drug treatments in the ANIT induced Primary Biliary Cirrhosis Model, utilizing cutting-edge techniques such as:
- General observations: Body weight, mortality rate, liver size, and stool consistency.
- Histological analysis: Liver tissue samples are examined for bile duct injury, inflammation, and fibrosis using Hematoxylin and Eosin (H&E) staining and Masson's Trichrome staining.
- Immunohistochemistry: Detection of immune cell infiltration (e.g., T-cells, macrophages) in liver tissue to assess the inflammatory response and the degree of fibrosis.
- Cytokine profiling (e.g., ELISA): Measurement of inflammatory cytokines such as TNF-α, IL-6, IL-1β, and other mediators involved in liver damage and fibrosis.
- Liver function markers: Serum biomarkers, including liver enzymes (ALT, AST), bilirubin levels, and alkaline phosphatase (ALP), to assess liver damage and cholestasis.
- Gene/protein expression profiling: Quantitative RT-PCR and Western blotting to evaluate the expression of fibrosis-related genes and proteins, such as collagen I, α-SMA, and fibronectin.
- Bile acid analysis: Quantification of serum bile acids to assess bile flow impairment and cholestasis progression.
In addition to these established techniques, our team is skilled in the development of novel experimental models tailored to your specific research goals. We help in experimental design, model selection, and data analysis, ensuring an efficient and effective approach to your project at every stage.
Related Services
In addition to ANIT induced models, we offer various other models that can also simulate cholestatic liver diseases, providing a broader understanding of therapeutic potential. We have extensive experience in using different induction methods for liver injury, ensuring flexibility in model selection to suit specific research goals. Our services are customized to meet your research needs.
- CCL4 induced Liver Fibrosis/Cirrhosis Model
- Thioacetamide (TAA) induced Liver Fibrosis/Cirrhosis Model
- Bile Duct Ligation (BDL) induced Primary Biliary Cirrhosis Model
- 3,5-Diethoxycarbonyl-1,4-Dihydrocollidine (DDC) induced Primary Biliary Cirrhosis Model
Advantages
- Expertise and Experience: Our team has extensive experience in developing and optimizing liver injury models, providing high-quality data for drug evaluation.
- Comprehensive Support: From experimental design to data analysis, we offer end-to-end support to ensure the success of your research.
- Customized Models: We offer tailored models, including modifications for specific disease pathways, enhancing the relevance to your research.
- Advanced Technology: We utilize cutting-edge techniques, such as cytokine profiling, histology, and gene expression analysis, to offer precise and reliable data.
- Flexible Services: Whether you need a single model or a comprehensive study, we provide flexible solutions to meet the scale and complexity of your project.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What are the advantages of using ANIT for the induction of primary biliary cirrhosis in animals?
ANIT effectively induces cholestatic liver injury and simulates key features of primary biliary cirrhosis, making it an ideal model for studying the disease.
-
2. How do I choose the right animal model for my research?
We offer personalized consultations to help you select the most suitable animal model based on your research objectives, ensuring a focused and efficient approach.
-
3. What kind of data can I expect from ANIT induced models?
You will receive comprehensive data on liver damage, inflammation, fibrosis, and drug efficacy, along with histological and biochemical analyses.
-
4. How long does it take to develop and establish an ANIT induced model?
The timeline for model establishment varies but typically takes 3-4 weeks, depending on the research scope and desired outcomes.
-
5. Can you help with experimental design and data analysis?
Yes, our scientific team is available to assist with experimental planning and detailed data analysis to ensure the best outcomes from your study.
Published Data
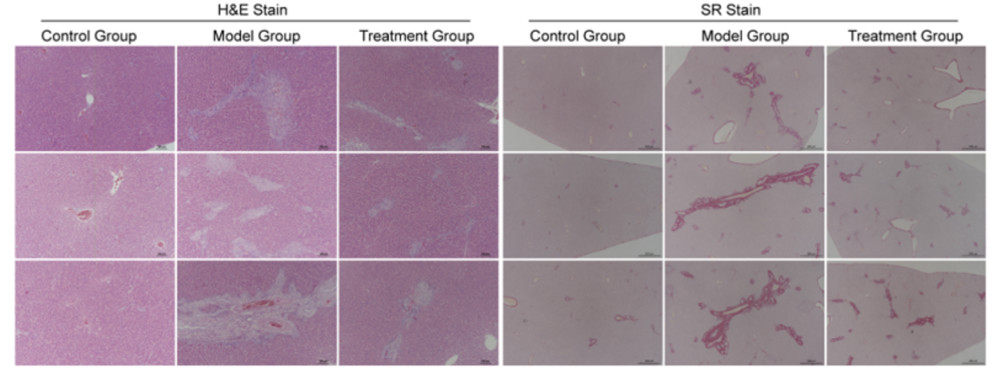 Fig. 1 The liver tissue pathology images of SD rats after 4 weeks of ANIT induction.
Fig. 1 The liver tissue pathology images of SD rats after 4 weeks of ANIT induction.
After 4 weeks of oral gavage administration of ANIT, significant pathological changes were observed in the liver tissue of SD rats. Compared to the blank control group, the model group showed hepatocellular edema, cytoplasmic vacuolization, disruption of the hepatic lobular structure, and localized hepatocyte necrosis and apoptosis under HE staining. Inflammatory cell infiltration was notably observed, especially in the perivenular regions. Additionally, liver sinusoids were dilated, and vascular congestion was prominent. Sirius Red staining revealed significant hepatic fibrosis, with collagen deposition mainly around the hepatic lobules and portal areas, indicating the onset of liver fibrosis. Oral gavage administration of ANIT effectively mimics both acute and chronic stages of chemical induced liver injury and fibrosis, making it a suitable model for studying liver disease mechanisms and screening potential therapeutic interventions.
For Research Use Only.
