Hepatitis B Humanized Mouse Modeling & Pharmacodynamics Service
Creative Biolabs offers a range of advanced animal models specifically designed to evaluate the efficacy of drugs targeting Hepatitis B. These models help assess therapeutic candidates by mimicking human disease progression, providing valuable insights into antiviral effectiveness and potential treatment strategies.
Introduction
Hepatitis B (HBV) is a viral infection that primarily affects the liver, caused by the Hepatitis B virus. It is transmitted through contact with the blood or other body fluids of an infected person, including through sexual contact, sharing needles, or from mother to child during birth. HBV can result in both acute and chronic infections, with chronic infection being particularly concerning. Chronic Hepatitis B (CHB) can lead to severe complications such as cirrhosis, liver failure, and hepatocellular carcinoma (liver cancer). The disease progresses in stages, from an acute phase, where symptoms may be mild or absent, to a chronic phase that may remain asymptomatic for years while causing gradual liver damage. Persistent infection occurs when the virus continues to replicate in the liver, evading the immune system. Current treatments, including nucleos(t)ide analogs and interferons, primarily aim to suppress the virus and prevent liver damage but do not offer a cure. With more than 250 million people worldwide living with chronic HBV, there remains a critical need for more effective treatments, particularly those that can provide a functional cure.
Disease Models and Applications
The Hepatitis B Humanized Mouse Model is established by humanizing the mouse liver with human hepatocytes, allowing the mice to support HBV infection. This model is constructed by transplanting human liver cells into immunodeficient mice, creating an environment where the virus can replicate in a manner similar to human infections. The model enables the study of HBV infection, immune responses, and the evaluation of therapeutic interventions. One key feature is its ability to replicate HBV's chronic infection cycle, providing a closer approximation of human disease than other animal models. However, there are limitations, including the complexity and cost of generating humanized mice, as well as variability in the success of hepatocyte engraftment. Despite these challenges, this model is invaluable for preclinical testing of antiviral agents and for studying the interaction between HBV and the human immune system, making it an essential tool in HBV research.
- Simulates: This model simulates Hepatitis B infection in humans, including both acute and chronic phases of disease. It mimics viral replication in hepatocytes, the progression of liver damage, and the immune response to the virus, offering a comprehensive view of HBV's impact on the liver.
- Evaluates Drugs: The Hepatitis B Humanized Mouse Model is used to evaluate antiviral treatments, including nucleos(t)ide analogs, immune modulators, and combination therapies. It helps assess the efficacy of these drugs in reducing viral load, improving liver function, and mitigating inflammation and fibrosis. This model is also used to test experimental therapies aimed at achieving a functional cure for HBV.
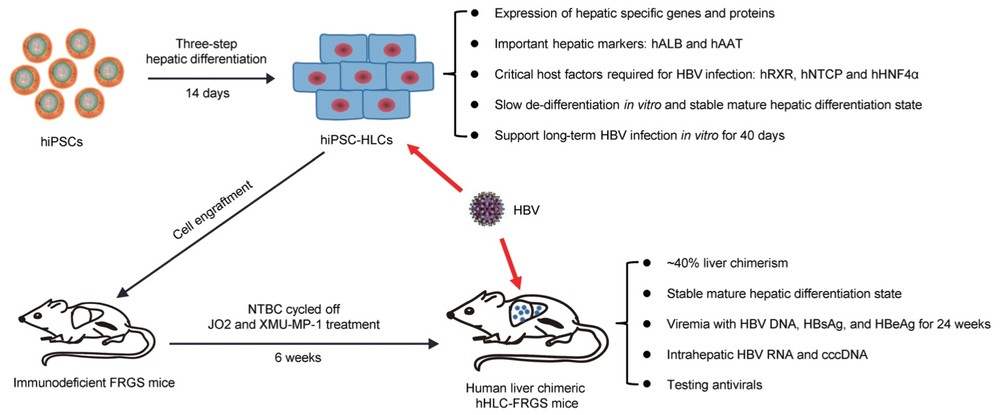 Fig. 1 Summary of hiPSC-HLCs and hHLC-FRGS mice supporting HBV infection.1
Fig. 1 Summary of hiPSC-HLCs and hHLC-FRGS mice supporting HBV infection.1
Measurements
For evaluating drug efficacy in this model, we utilize a variety of advanced techniques, including:
- General observations: Body weight, liver size, and signs of jaundice or other systemic issues.
- Histopathology: Liver tissue analysis for inflammation, fibrosis, and viral replication.
- Immunohistochemistry: Detection of immune cell infiltration (e.g., T-cells, macrophages) in liver tissues.
- Cytokine profiling: Measurement of inflammatory cytokines (e.g., TNF-α, IL-6, IL-1β) using ELISA.
- HBV DNA quantification: Real-time PCR to measure viral load in the serum and liver tissue.
- Liver function tests: Assessment of serum biomarkers, such as ALT, AST, and bilirubin levels.
- Gene/protein expression: Profiling viral and host response genes using RT-qPCR and Western blot techniques.
Additionally, our team provides full support in experimental design, model selection, and data analysis to ensure the model is tailored to specific research needs.
Related Services
Beyond the humanized mouse model, we offer alternative methods for inducing Hepatitis B in mice using different human liver cell lines or viral vectors. These models provide additional options for testing drugs under varying conditions of immune response and viral replication.
- AAV/HBV induced Chronic HBV Infection Model
- HBV Transgenic Mouse Model
- Hydrodynamic Injection HBV Model
- Chronic Hepatitis B Woodchuck Model
Advantages
- Comprehensive Expertise: Our team brings years of specialized experience in Hepatitis B research, providing unparalleled insights into disease mechanisms and therapeutic testing.
- Tailored Solutions: We offer customizable models and research plans that are tailored to your specific project needs, ensuring that your research objectives are met with precision.
- Cutting-Edge Technology: We use state-of-the-art technologies and the latest methodologies to generate high-quality, reliable data that meets the highest scientific standards.
- Robust Animal Models: Our Hepatitis B models are rigorously developed to simulate human infection, providing a true-to-life representation of HBV infection and progression, crucial for accurate preclinical testing.
- Comprehensive Support: From model selection to data analysis, we provide full-service support, guiding you every step of the way to ensure your project's success.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What makes the humanized mouse model ideal for Hepatitis B research?
The humanized mouse model closely mimics human HBV infection, allowing for realistic studies of viral replication, immune response, and drug efficacy.
-
2. How does this model simulate HBV infection in humans?
By transplanting human hepatocytes into immunocompromised mice, the model provides an environment where HBV can replicate and interact with the human immune system, resembling the chronic infection cycle in humans.
-
3. What types of drugs can be tested using this model?
Antiviral agents, immune modulators, and combination therapies aimed at suppressing or eradicating HBV infection can be tested using this model.
-
4. Are there any limitations to using this model?
While highly valuable, the model can be complex and costly to develop, and there can be variability in hepatocyte engraftment success, which may affect the consistency of results.
-
5. Can this model be used for studying HBV-related liver diseases?
Yes, this model is ideal for studying not only HBV infection but also its progression to liver inflammation, fibrosis, and hepatocellular carcinoma.
Published Data
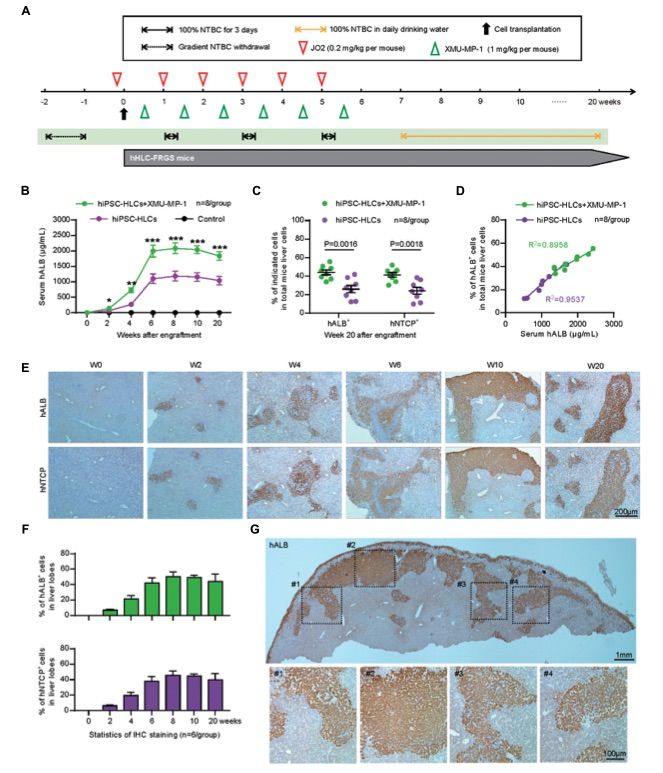 Fig. 2 Generation and characterization of human liver chimeric hHLC-FRGS mice.1
Fig. 2 Generation and characterization of human liver chimeric hHLC-FRGS mice.1
A chimeric human liver mouse model was established to investigate HBV pathogenesis in vivo by engrafting hiPSC-derived human liver cells (hiPSC-HLCs) into FRGS mice via splenic injection. Liver injury was induced using NTBC cycling and the mouse CD95 antibody JO2, which selectively killed a portion of the mouse liver cells, creating space for the engrafted hiPSC-HLCs to proliferate. To enhance the expansion of the implanted hiPSC-HLCs, the mice were treated weekly with the small molecule XMU-MP-1, previously shown to augment hepatocyte expansion. At week 6 post-engraftment, the serum hALB concentration in XMU-MP-1-treated hHLC-FRGS mice was 1997.2 ± 195.6 mg/mL, and this concentration was maintained above 1839.8 ± 140.9 mg/mL until week 20. In contrast, the serum hALB concentration in untreated mice was 1099.6 ± 154.6 mg/mL at week 6 and remained below 1041.4 ± 136.2 mg/mL throughout the study period. The differences in serum hALB concentrations between the treated and untreated groups were statistically significant (Figure 2A). Further analysis of the chimeric liver at week 20 post-engraftment revealed the proportion of hiPSC-HLCs producing hALB and hNTCP. Flow cytometry (FACS) showed that 44.1 ± 2.6% of liver cells in XMU-MP-1-treated mice were positive for hALB, compared to 25.9 ± 3.9% in untreated mice (Figure 2B). The percentage of hNTCP-positive cells was similar between treated and untreated mice. A significant linear correlation was observed between the serum hALB levels and the proportion of hALB-positive cells in the liver (Figure 2C). Immunohistochemical (IHC) assays of liver tissue collected from week 0 to 20 post-engraftment confirmed the robust expansion of hiPSC-HLCs in XMU-MP-1-treated mice, with hALB-positive cells maintained at 41.9 ± 6.8% through week 20 (Figure 2D). Statistical analysis of multiple liver sections from various lobes confirmed the stable chimerism of the implanted hiPSC-HLCs (Figures 2E, F).
Reference
- Yuan, Lunzhi et al. "A Chimeric Humanized Mouse Model by Engrafting the Human Induced Pluripotent Stem Cell-Derived Hepatocyte-Like Cell for the Chronic Hepatitis B Virus Infection." Frontiers in microbiology vol. 9 908. 8 May. 2018, DOI:10.3389/fmicb.2018.00908. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
