Monosodium Urate (MSU) induced Gout Modeling & Pharmacodynamics Service
Creative Biolabs provides advanced services for modeling and evaluating drug efficacy in MSU induced gout, offering robust disease models and personalized pharmacodynamics assessments to help in therapeutic development. We support the development of new treatments with reliable, reproducible animal models and tailored testing protocols.
Introduction
Gout is a form of inflammatory arthritis that occurs when uric acid crystals accumulate in the joints, leading to sudden and severe pain, swelling, and redness. The disease is often associated with hyperuricemia, a condition where there is an excess of uric acid in the bloodstream, which crystallizes and deposits in the joints, particularly affecting the big toe. Gout attacks can be triggered by factors such as alcohol consumption, high-purine diets, dehydration, and certain medications. Chronic gout can result in joint damage, tophi (uric acid crystal deposits under the skin), and kidney stones. Gout is a major health issue, affecting millions globally, with a significant impact on the quality of life.
Disease Models and Applications
The Monosodium Urate (MSU) induced Gout Model is widely used in preclinical research to study the pathogenesis of gout and evaluate potential therapies. The model is typically induced by the injection of MSU crystals into the joint cavity, such as the ankle or knee, of rodents. This model mimics the acute inflammatory response seen in human gout, including the characteristic swelling and pain. One of the key advantages is its ability to replicate the acute flare-ups associated with the disease, making it ideal for testing anti-inflammatory and urate-lowering drugs. However, the model also has limitations, such as the short duration of the inflammation and the lack of chronicity seen in human gout. Despite these limitations, MSU induced models remain valuable tools for evaluating drugs aimed at preventing or treating gout.
- Simulates: MSU induced gout model effectively simulates the acute inflammatory responses seen in human gout, especially the deposition of MSU crystals in joints, leading to severe inflammation and pain. This model helps in understanding the mechanisms of disease onset and flare-ups.
- Evaluates Drugs: This model is used to evaluate anti-inflammatory drugs, urate-lowering agents, and novel gout therapeutics. These include NSAIDs, corticosteroids, colchicine, and biologics like IL-1 inhibitors. Our platform offers comprehensive testing to determine the efficacy of these drugs in controlling inflammation and preventing future gout attacks.
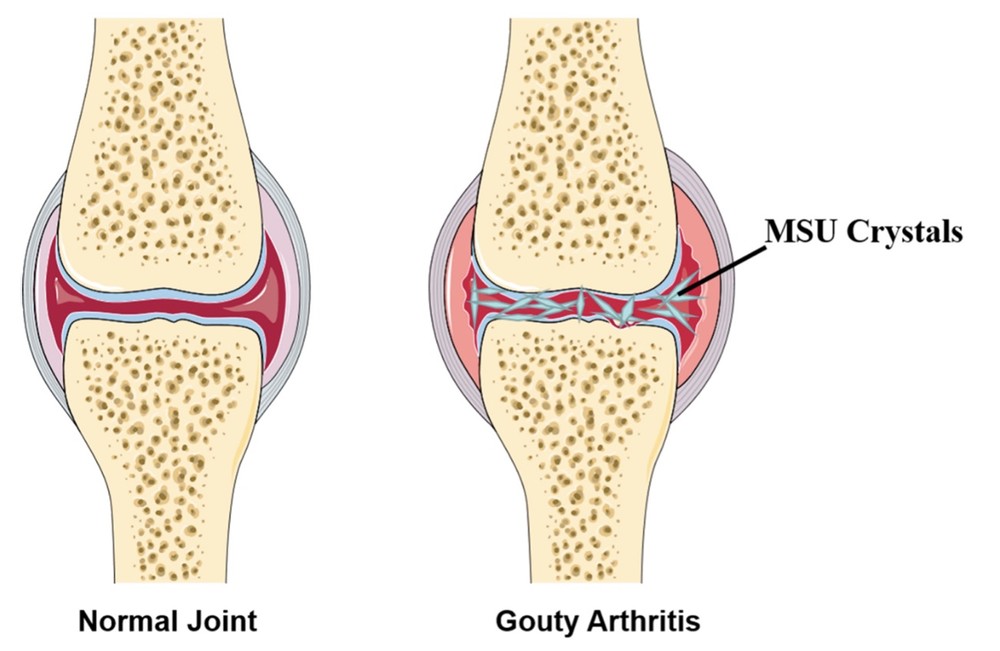 Fig. 1 Abridged general view of normal joint and Gouty Arthritis (GA).1
Fig. 1 Abridged general view of normal joint and Gouty Arthritis (GA).1
Measurements
We offer a variety of measurements for evaluating drug efficacy in the MSU induced Gout Model, utilizing advanced technologies, including but not limited to:
- General observations: Joint swelling, redness, pain behavior (e.g., limping, licking).
- Histological analysis: Infiltration of immune cells (e.g., neutrophils, macrophages) in joint tissues.
- Cytokine profiling (e.g., ELISA): Expression levels of inflammatory mediators such as IL-1β, IL-6, and TNF-α.
- Gene/protein expression profiling: RT qPCR and Western blot techniques to evaluate key inflammatory markers.
- X-ray imaging: Assess joint damage and bone erosion, especially in chronic gout models.
Our expert team helps design experiments and analyze data to ensure that measurements are tailored to specific research needs.
Advantages
- Cutting-Edge Expertise: Our team has extensive experience in creating and optimizing gout models, ensuring precise and relevant research outcomes.
- Tailored Approaches: We provide customized solutions based on your specific research needs, ensuring that models and testing protocols align with your objectives.
- Comprehensive Support: From model selection to experimental design and data analysis, we offer full-service support throughout the research process.
- Advanced Technology: We utilize the latest technologies, including imaging, histology, and gene expression profiling, to provide detailed and reliable results.
- Efficient Turnaround: We offer fast and reliable results, allowing you to progress with your research and drug development timelines.
- Regulatory Compliance: Our services adhere to the highest standards of research integrity, ensuring that your studies meet all relevant regulatory guidelines.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What types of models do you offer for gout research?
We provide a range of models, including MSU induced acute gout models, chronic gout models, and hyperuricemia models, each designed to meet specific research needs.
-
2. How can your services help with drug development?
Our models are designed to evaluate the efficacy and safety of potential gout treatments, offering a reliable preclinical platform for testing new therapies.
-
3. Do you offer chronic gout models?
Yes, we offer modified models that mimic the long-term inflammatory process and joint damage associated with chronic gout.
-
4. What is the typical timeline for results?
Acute inflammation can be observed within 24–48 hours, while chronic models may take longer. We provide an efficient timeline based on your project needs.
-
5. Are the models customizable?
Yes, our gout models can be customized to suit specific research questions, including variations in disease severity, treatment protocols, and outcome measures.
Published Data
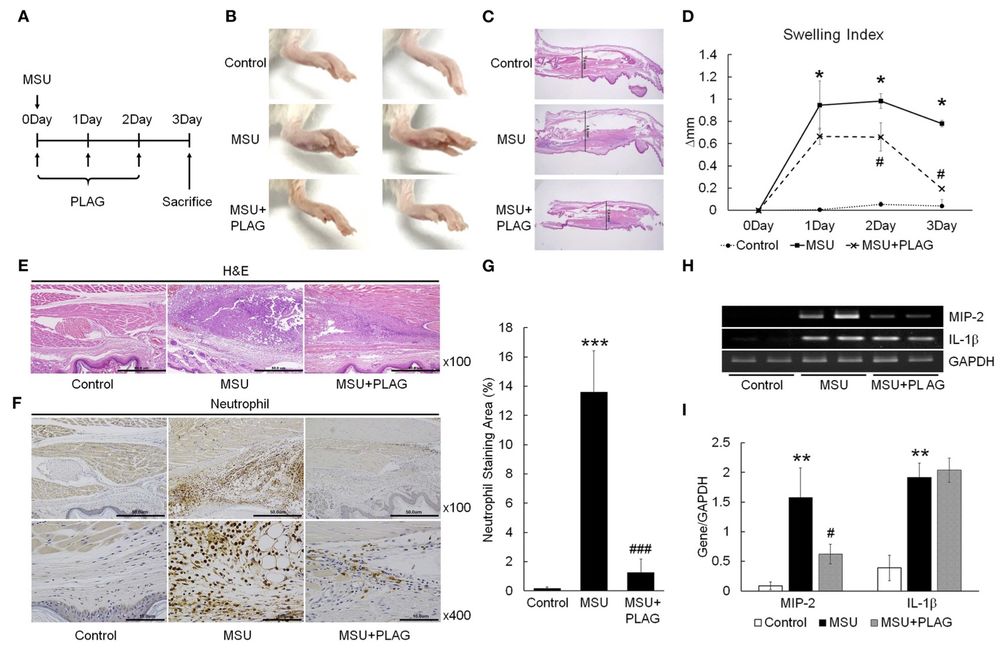 Fig. 2 PLAG alleviates MSU induced gouty inflammation by reducing neutrophil infiltration in gouty lesions.2
Fig. 2 PLAG alleviates MSU induced gouty inflammation by reducing neutrophil infiltration in gouty lesions.2
The effect of PLAG on reducing gouty inflammation was evaluated using an MSU induced acute gout animal model (Figure 2A). Swelling is a prominent symptom of MSU induced gout and can be easily detected through visual inspection. Paw swelling was observed within 1 day following MSU injection into the mouse footpad, with swelling persisting for 3 days. Upon administering PLAG to MSU-treated mice, a faster recovery of footpad swelling was observed compared to untreated MSU mice (Figure 2B). Paw swelling was also assessed in dissected tissues, where the thickness was measured over a 3-day period. The results showed that in untreated MSU mice, footpad swelling remained for 3 days. However, in the PLAG-treated group, swelling was less severe and resolved within the same time frame (Figures 2C, D). Neutrophil infiltration in the MSU-injected areas was evident through immunohistochemical staining. Notably, neutrophil infiltration was absent in the footpad tissues of PLAG-treated mice (Figures 2E–G). The expression of chemokine MIP-2, a key factor for neutrophil recruitment in MSU-injected tissues, was assessed via RT-PCR. MIP-2 expression was elevated in the tissues of MSU-treated mice, but it was significantly reduced in the tissues of mice treated with PLAG (Figures 2H, I). Although IL-1β expression was induced in the MSU-treated tissues, PLAG had no noticeable effect on modulating IL-1β levels in the MSU-injected tissues. Overall, these findings suggest that PLAG alleviated acute gouty inflammation induced by MSU through the modulation of neutrophil migration, with this effect occurring within a 3-day period.
References
- Lyu, Shang et al. "LC-MS Analysis of Serum for the Metabolomic Investigation of the Effects of Pulchinenoside b4 Administration in Monosodium Urate Crystal induced Gouty Arthritis Rat Model." Molecules (Basel, Switzerland) vol. 24,17 3161. 30 Aug. 2019, DOI:10.3390/molecules24173161. Distributed under an Open Access license CC BY 4.0, without modification.
- Shin, Su-Hyun et al. "1-Palmitoyl-2-Linoleoyl-3-Acetyl-rac-Glycerol (PLAG) Mitigates Monosodium Urate (MSU) induced Acute Gouty Inflammation in BALB/c Mice." Frontiers in immunology vol. 11 710. 24 Apr. 2020, DOI:10.3389/fimmu.2020.00710. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
