Hydrodynamic Injection HBV Modeling & Pharmacodynamics Service
Creative Biolabs offers a range of well-established animal models, including HBV transgenic mice and hydrodynamic injection models, to evaluate the efficacy of antiviral treatments, providing reliable preclinical data to support your research in Hepatitis B drug development.
Introduction
Hepatitis B (HBV) is a viral infection that primarily affects the liver, causing both acute and chronic diseases. The virus is transmitted through contact with infected blood, semen, or other body fluids. Acute infection can cause symptoms like jaundice, fatigue, and abdominal pain, and in some cases, can resolve without long-term effects. However, in many individuals, HBV becomes chronic, leading to serious complications such as cirrhosis, liver failure, and hepatocellular carcinoma (liver cancer). Chronic HBV infection often progresses silently, without obvious symptoms, for decades, making it challenging to diagnose early. The global prevalence of chronic HBV is high, with an estimated 296 million people infected worldwide. While antiviral treatments can suppress viral replication, they do not provide a cure, and long-term management is necessary to prevent liver damage. Early diagnosis and effective treatments are essential in preventing complications, and animal models play a crucial role in developing and testing these therapies.
Disease Models and Applications
The Hydrodynamic Injection HBV Model involves the introduction of HBV plasmid DNA into the liver of rodents through a rapid injection into the tail vein. This method causes the DNA to transiently express HBV antigens in the liver, simulating HBV infection. The model has the advantage of providing a rapid onset of HBV infection, making it ideal for testing antiviral drugs and therapeutic vaccines. It is also less expensive and faster compared to other chronic HBV models, such as transgenic mice. However, the model's transient nature, meaning the virus does not persist long-term like in human infection, limits its ability to fully replicate chronic HBV disease. Despite this limitation, the model remains useful for evaluating the immediate immune response and antiviral drug effects. The hydrodynamic injection method is particularly valuable for assessing acute-phase interventions and understanding early viral dynamics in liver tissues.
- Simulates: This model simulates an acute HBV infection in rodents, allowing researchers to study the initial stages of viral replication and immune responses in the liver.
- Evaluates Drugs: The hydrodynamic injection HBV model is used to evaluate antiviral drugs, immune modulators, and vaccine candidates. It helps assess the effectiveness of treatments in reducing viral load, preventing liver damage, and stimulating immune responses.
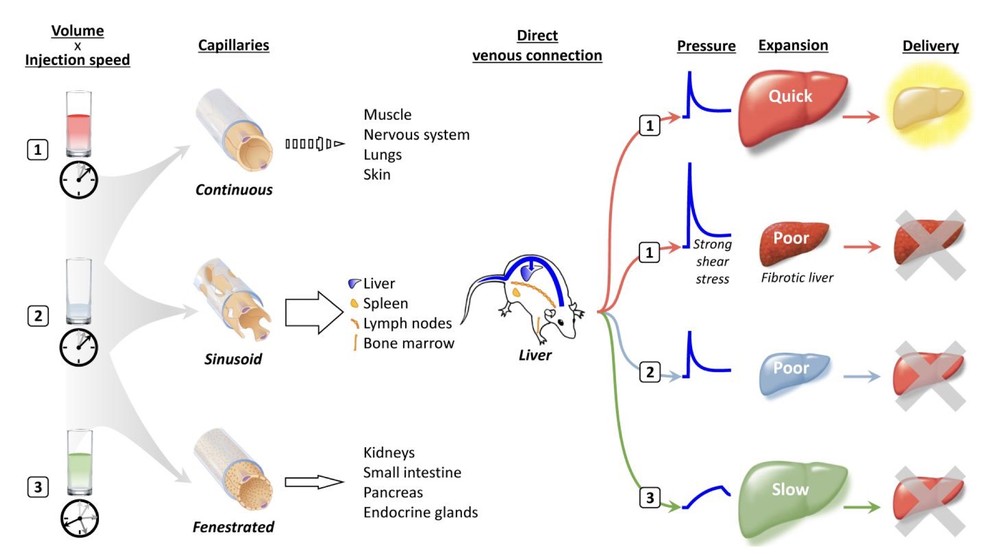 Fig.1 Traverse of hydrodynamic impact from injection to gene transfer sites.1
Fig.1 Traverse of hydrodynamic impact from injection to gene transfer sites.1
Measurements
We offer a range of measurements for evaluating drug efficacy in the Hydrodynamic Injection HBV Model, utilizing cutting-edge technologies, including but not limited to:
- General observations: Body weight, mortality rate, serum HBV markers (e.g., HBsAg, HBeAg).
- Immunohistochemistry: Detection of HBV antigens and immune cell infiltration (e.g., CD4+, CD8+ T-cells, macrophages) in liver tissues.
- Cytokine profiling (e.g., ELISA): Measurement of inflammatory cytokines such as TNF-α, IL-6, IL-1β, and IFN-γ.
- Liver function biomarkers: Serum levels of liver enzymes (ALT, AST), bilirubin, and albumin.
- Gene/protein expression analysis: RT-qPCR and Western blot techniques to examine HBV gene expression and immune response markers.
These assessments allow a comprehensive evaluation of drug effects on viral replication, immune responses, and liver health. Additionally, our expert team provides support in experimental design, model selection, and data interpretation, ensuring tailored solutions for your research needs.
Related Services
In addition to the Hydrodynamic Injection HBV Model, we also offer other methods for inducing HBV infection in animals, such as the HBV transgenic mouse model and the hydrodynamic injection of hepatocytes. These models provide alternative methods for assessing therapeutic candidates, giving you a comprehensive view of potential treatments.
- AAV/HBV induced Chronic HBV Infection Model
- HBV Transgenic Mouse Model
- Hepatitis B Humanized Mouse Model
- Chronic Hepatitis B Woodchuck Model
Advantages
- Expertise: Our team has extensive experience in designing and providing customized animal models to meet the specific needs of your research.
- Advanced Techniques: We utilize state-of-the-art technologies, including immunohistochemistry, cytokine profiling, and gene expression analysis, to deliver comprehensive results.
- Flexible Solutions: Whether you require a rapid screening model or a long-term chronic infection model, we offer flexibility to meet your study's timeline and goals.
- Collaborative Approach: We work closely with you throughout the study, offering guidance in experimental design, model selection, and data analysis for successful outcomes.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What is the primary advantage of using the Hydrodynamic Injection HBV Model?
This model allows for rapid viral infection, providing a quick and cost-effective way to evaluate acute-phase interventions and antiviral drug efficacy.
-
2. Can the Hydrodynamic Injection HBV Model replicate chronic HBV infection?
No, this model primarily simulates an acute phase of infection. However, it is valuable for testing antiviral drugs and immune responses in the early stages of infection.
-
3. How do the results from this model translate to human treatment?
While this model does not fully replicate chronic infection, it provides important insights into initial immune responses and antiviral drug effects, which are critical for developing treatments for chronic HBV.
-
4. Can the model be used to test therapeutic vaccines?
Yes, the Hydrodynamic Injection HBV Model is useful for evaluating therapeutic vaccines by assessing immune responses to HBV antigens expressed in the liver.
-
5. What types of measurements are taken in this model?
We assess viral load, liver function, immune responses, and tissue damage markers using a variety of methods such as ELISA, immunohistochemistry, and RT-qPCR.
Published Data
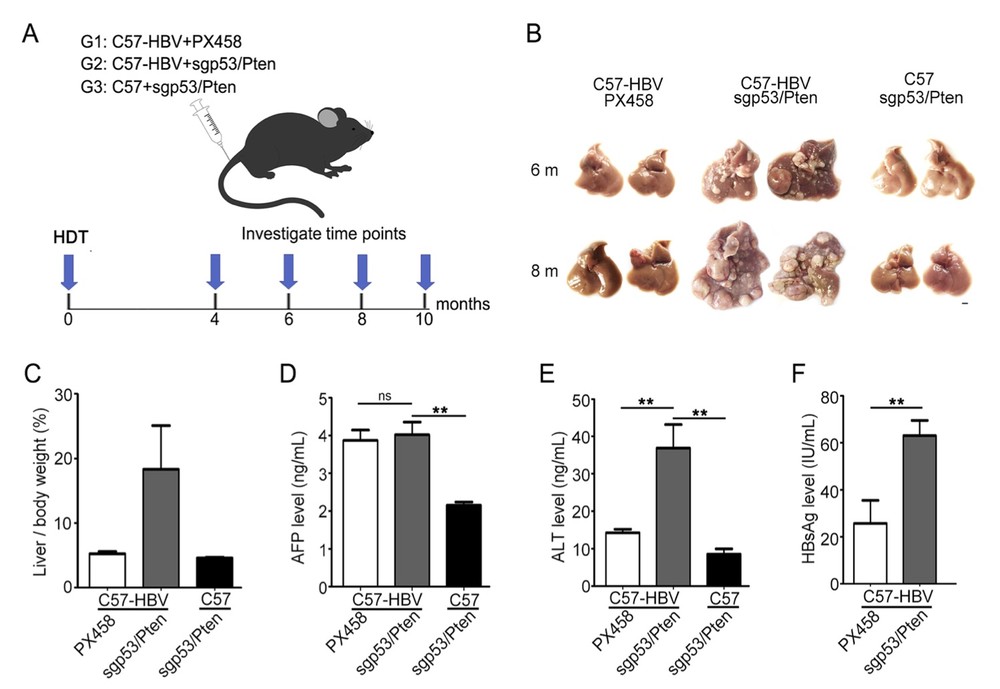 Fig. 2 Hydrodynamic injection of the dual sgRNA cassette promoted accelerated tumor development in HBV transgenic mice.2
Fig. 2 Hydrodynamic injection of the dual sgRNA cassette promoted accelerated tumor development in HBV transgenic mice.2
The sgp53/Pten dual cassette plasmids were administered to the livers of 5–7 week old C57-HBV mice via hydrodynamic tail vein injection. A control group consisting of HBV transgenic mice of the same strain, age, and gender was injected with PX458 plasmids. Additionally, wild-type C57BL/6 mice injected with sgp53/Pten dual cassette plasmids served as a control for non-HBV infection (Fig. 2A). Macroscopic tumors appeared as early as four months after injection in the C57-HBV mice treated with sgp53/Pten dual cassette. Over time, the size of the tumors progressively increased, with tumor-burdened livers exhibiting more and larger nodules at 6 and 8 months post-injection (Fig. 2B). In contrast, none of the control groups developed detectable nodules by 6 and 8 months, although one C57 mouse injected with sgp53/Pten dual cassette developed a small liver tumor at 10 months. Quantitative analysis revealed that the ratio of liver weight to body weight in the C57-HBV mice injected with sgp53/Pten dual cassette plasmids was significantly higher at 8 months post-injection compared to control mice (20% vs. 5%) (Fig. 2C). These findings demonstrated that somatic gene dysfunction, achieved by hydrodynamic tail vein injection of Cas9 and p53/Pten dual gRNAs, accelerates tumor formation in HBV transgenic mice. At 8 months post-injection, the serum AFP level in the tumor-bearing C57-HBV mice was significantly elevated compared to C57 mice injected with sgp53/Pten dual cassette plasmids but was comparable to the level in the HBV-C57 mice injected with PX458 plasmids (Fig. 2D). Additionally, serum ALT levels, a marker of hepatocyte damage, were markedly higher in the tumor-bearing C57-HBV mice compared to those of the tumor-free C57 or C57-HBV control mice (Fig. 2E). The serum HBsAg level was also significantly elevated in the tumor-bearing C57-HBV mice injected with sgp53/Pten dual cassette plasmids (Fig. 2F). Collectively, these results suggest that the sgp53/Pten dual cassette treatment induces severe liver damage in HBV transgenic mice.
References
- Suda, Takeshi et al. "Hydrodynamic Delivery: Characteristics, Applications, and Technological Advances." Pharmaceutics vol. 15,4 1111. 31 Mar. 2023, DOI:10.3390/pharmaceutics15041111. Distributed under an Open Access license CC BY 4.0, without modification.
- Liu, Yongzhen et al. "CRISPR/Cas9-mediated p53 and Pten dual mutation accelerates hepatocarcinogenesis in adult hepatitis B virus transgenic mice." Scientific Reports vol. 7,1 2796. 5 Jun. 2017, DOI:10.1038/s41598-017-03070-8. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
