HBV Transgentic & Carbon Tetrachloride (CCL₄) induced Liver Modeling & Pharmacodynamics Service
Creative Biolabs offers a range of sophisticated animal models designed to evaluate the efficacy of drugs targeting HBV infection and liver fibrosis. These models provide valuable insights into the disease‘s progression and the potential for therapeutic interventions. Our services include comprehensive preclinical studies that guide the development of new treatments and drug screening for HBV-related liver diseases.
Introduction
Hepatitis B virus (HBV) infection is a global health concern, affecting over 290 million people worldwide. Chronic HBV infection can lead to serious liver complications, including cirrhosis, liver failure, and hepatocellular carcinoma (HCC). The virus primarily targets hepatocytes, and persistent infection induces inflammation, immune responses, and hepatic injury. Over time, chronic HBV infection triggers liver fibrosis, a progressive accumulation of extracellular matrix proteins and scar tissue, which impairs liver function. Fibrosis is a significant predictor of disease progression and is typically staged from mild to severe, with advanced fibrosis or cirrhosis increasing the risk of HCC. The pathogenesis of HBV-related liver fibrosis involves both viral factors and host immune responses. As fibrosis advances, it can lead to irreversible liver damage, emphasizing the need for early intervention. Management of HBV-related liver fibrosis focuses on antiviral therapy to reduce viral replication and fibrosis progression. However, effective treatments to reverse liver fibrosis remain a critical area of research.
Disease Models and Applications
The combined HBV transgenic and CCl4 induced liver fibrosis model is a highly effective tool for studying the pathophysiology of liver fibrosis in the context of chronic HBV infection. This model is constructed by administering carbon tetrachloride (CCl4), a potent hepatotoxic agent, to HBV-transgenic mice, which express HBV antigens and replicate the viral infection. CCl4 induces liver injury, stimulating fibrosis progression, and the model mimics the fibrosis observed in chronic HBV patients. One of the model's advantages is its ability to simulate both HBV infection and the liver damage resulting from environmental insults like alcohol or drugs. However, a limitation is that it may not fully replicate the complexity of human immune responses to HBV infection. Despite this, the model is invaluable for testing antiviral drugs, anti-fibrotic agents, and therapeutic strategies aimed at reversing liver damage caused by HBV.
- Simulates: This model simulates the progression of liver fibrosis in chronic HBV infection, including the inflammatory response, hepatocellular injury, and fibrogenesis, similar to the conditions observed in human patients.
- Evaluates Drugs: The model is useful for evaluating a wide range of therapeutic compounds, including antiviral drugs aimed at inhibiting HBV replication, anti-fibrotic agents targeting hepatic stellate cells, and immune-modulating treatments designed to alleviate inflammation and fibrosis.
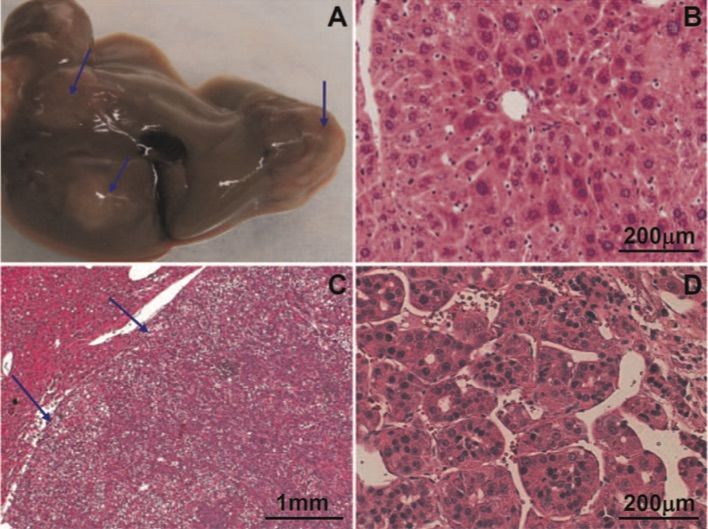 Fig. 1 Hepatocellular neoplasms in Tg05 wildtype HBV transgenic mice.1
Fig. 1 Hepatocellular neoplasms in Tg05 wildtype HBV transgenic mice.1
Measurements
We offer a range of advanced measurements to evaluate the efficacy of drug treatments in the HBV Transgenic and CCl4 induced Liver Fibrosis mouse model. These include:
- General observations: Body weight, liver function, mortality rates, and signs of fibrosis (e.g., ascites, jaundice).
- Histopathology: Liver tissue examination for fibrosis, hepatocyte necrosis, and inflammation using Hematoxylin and Eosin (H&E) staining.
- Immunohistochemistry: Immune cell infiltration (e.g., T-cells, macrophages) and HBV antigen expression in liver tissues.
- Cytokine profiling: Measurement of inflammatory cytokines (e.g., TNF-α, IL-6, IL-1β) using ELISA.
- Liver enzymes and biomarkers: Serum analysis for ALT, AST, and bilirubin levels to monitor liver damage.
- Gene and protein expression profiling: RT-qPCR and Western blot to quantify the expression of fibrosis-related genes such as collagen I, α-SMA, and TGF-β.
Our team is equipped to assist with experimental design, model selection, and data interpretation, ensuring a tailored and comprehensive approach to your research.
Advantages
- Comprehensive Expertise: We offer an array of specialized models for studying HBV-related liver diseases, with extensive experience in preclinical testing.
- Customizable Approaches: Tailor-made solutions to meet your specific research needs, including personalized experimental design and data analysis.
- Cutting-Edge Technologies: Utilize the latest advancements in molecular techniques, including qPCR, Western blotting, and advanced imaging.
- Reliable and Reproducible Results: Our models provide consistent and reproducible outcomes to support robust scientific findings.
- Rapid Turnaround: Efficient project management ensures that you receive timely results without compromising on quality.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What is the HBV Transgenic and CCl4 induced Liver Fibrosis mouse model?
It’s a rodent model used to study liver fibrosis caused by chronic HBV infection and environmental toxins. CCl4 induces liver injury and fibrosis in HBV-transgenic mice, mimicking the disease progression seen in humans.
-
2. How long does it take to establish this model?
The induction of fibrosis typically takes 4-6 weeks, depending on the CCl4 administration regimen and the desired stage of fibrosis.
-
3. What types of drugs can be tested in this model?
This model is ideal for testing antiviral agents, anti-fibrotic drugs, immune modulators, and liver protectants.
-
4. Can this model simulate both viral and environmental liver damage?
Yes, the model simulates both HBV infection and liver fibrosis caused by environmental factors like CCl4, making it a comprehensive tool for evaluating treatment options.
-
5. Do you provide customized experimental design services?
Yes, our scientific team is available to assist with model selection, experimental design, and data interpretation to meet the unique needs of your research.
Published Data
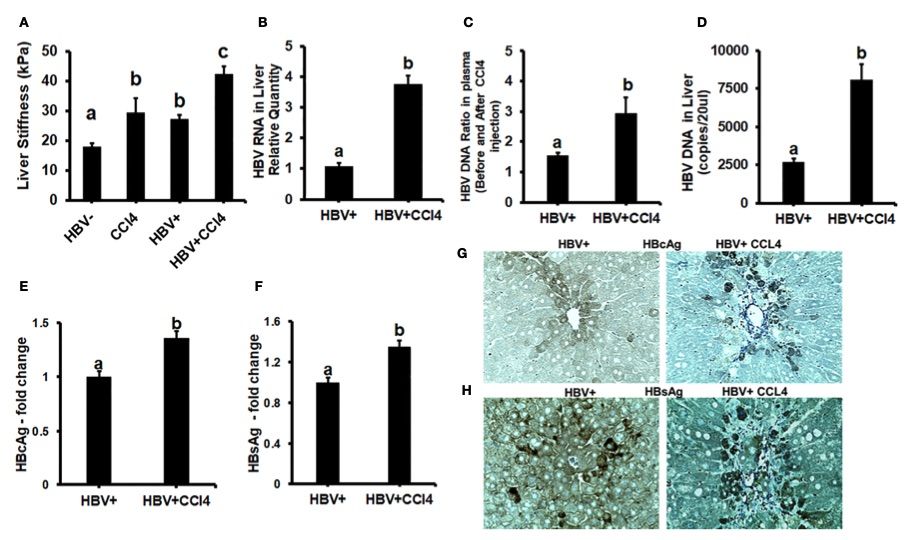 Fig. 2 Increased liver tissue stiffness regulates HBV replication/enhanced expression of HBV markers in livers of CCl4-administered HBV+ transgenic mice.2
Fig. 2 Increased liver tissue stiffness regulates HBV replication/enhanced expression of HBV markers in livers of CCl4-administered HBV+ transgenic mice.2
As shown in Figure 2A, ex vivo liver tissue stiffness in control mice is measured at 17 kPa. This value may be influenced by alterations in mechanical properties due to factors such as perfusion pressure, tissue degradation, and boundary conditions when compared to the in vivo environment. Additionally, the study highlights that the mechanical properties of tissues change rapidly within minutes postmortem. To minimize this effect, all conditions were processed on the same day to ensure consistency in stiffness measurement and comparison. Based on these considerations, liver tissue stiffness in wild-type control mice is regarded as representative of healthy liver stiffness, while the increase in stiffness observed in CCl4-treated mice corresponds to fibrotic changes. Interestingly, control HBVTg mice exhibited higher liver tissue stiffness compared to wild-type controls. To assess whether this increase in liver stiffness contributes to the upregulation of HBV infection markers in CCl4-treated HBVTg mice, HBV RNA and DNA levels were measured in mouse livers, and HBV DNA in serum was quantified using the COBAS Amplicor HBV monitor test. Furthermore, immunohistochemical analysis of HBcAg and HBsAg expression in the liver was performed (Figures 2B-H).
References
- Kosinska, Anna D et al. "Therapeutic vaccination and immunomodulation in the treatment of chronic hepatitis B: preclinical studies in the woodchuck." Medical microbiology and immunology vol. 204,1 (2015): 103-14. DOI:10.1007/s00430-014-0379-5. Distributed under an Open Access license CC BY 4.0, without modification.
- Bybee, Grace et al. "Increased liver stiffness promotes hepatitis B progression by impairing innate immunity in CCl4 induced fibrotic HBV+ transgenic mice." Frontiers in immunology vol. 14 1166171. 3 Aug. 2023, DOI:10.3389/fimmu.2023.1166171. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
