Acetaminophen (APAP) induced Acute Liver Injury Modeling & Pharmacodynamics Service
Creative Biolabs offers a range of well-established acute liver injury models to assess the efficacy of therapeutic drugs. These models help simulate human liver injury and are instrumental in testing drug safety and effectiveness.
Introduction
Acute liver injury (ALI) is a severe condition that can lead to liver failure, characterized by rapid liver dysfunction caused by various factors such as viral infections, alcohol consumption, toxins, and certain medications. It presents with symptoms like jaundice, liver tenderness, and abnormal liver function tests. ALI can progress to acute liver failure if not treated in time, and it poses a significant risk to patient survival. The underlying mechanisms involve hepatocyte necrosis, inflammation, and oxidative stress, all of which contribute to liver dysfunction. Various models have been developed to replicate ALI in animal systems, providing critical insights into disease pathogenesis and therapeutic strategies. These models are essential for evaluating the effects of potential treatments and understanding how drugs influence liver recovery or deterioration.
Disease Models and Applications
The Acetaminophen (APAP) induced Acute Liver Injury (ALI) model is created by administering a toxic dose of acetaminophen, typically by oral or intraperitoneal injection, to rodents. This model closely replicates the pathological features of human drug induced liver injury, including hepatocyte necrosis, inflammation, and oxidative stress. After administration, the liver undergoes cellular damage due to the accumulation of toxic metabolites, especially NAPQI, which depletes glutathione levels and induces hepatotoxicity. This model is useful for studying the underlying mechanisms of drug induced liver injury, such as mitochondrial dysfunction, oxidative stress, and inflammation. It is commonly used to assess potential hepatoprotective compounds, anti-inflammatory drugs, and agents that modulate oxidative stress. However, a limitation of the APAP model is that it primarily induces acute liver injury and may not fully mimic chronic liver diseases such as cirrhosis or non-alcoholic fatty liver disease (NAFLD). Despite this, the APAP induced model remains one of the most widely used preclinical models for liver toxicity testing.
- Simulates: The APAP induced model simulates drug induced liver injury, which shares key features with human cases of acute liver failure due to drug toxicity, particularly hepatocyte necrosis and inflammation.
- Evaluates Drugs: This model is used to evaluate drugs aimed at protecting the liver from damage, such as hepatoprotective agents, anti-inflammatory drugs, antioxidants, and compounds targeting oxidative stress and apoptosis.
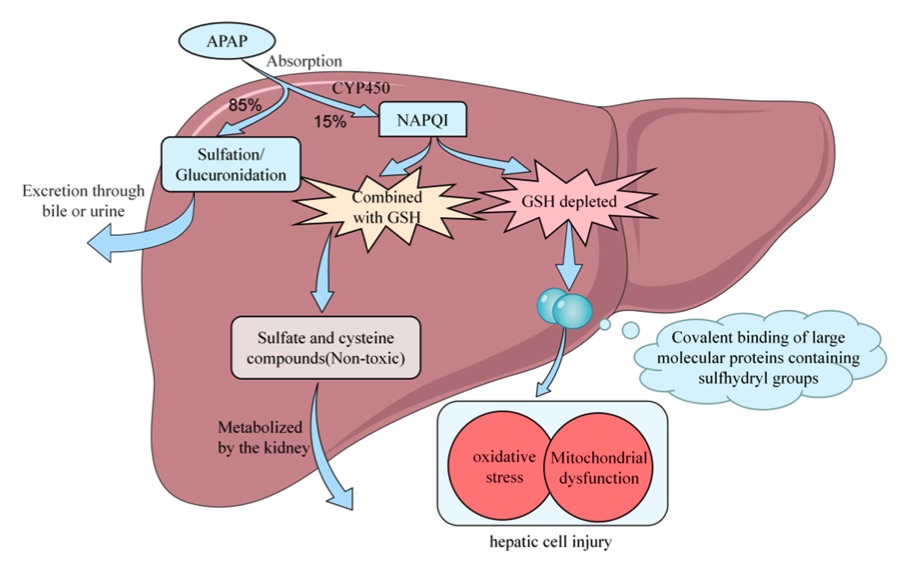 Fig. 1 Schematic representation of APAP metabolism in the liver.1
Fig. 1 Schematic representation of APAP metabolism in the liver.1
Measurements
For the Acetaminophen (APAP) induced Acute Liver Injury Model, we offer a variety of measurements to evaluate drug efficacy, including:
- General observations: Body weight, mortality rate, survival time, and signs of liver failure such as jaundice.
- Histological Analysis: Liver tissue sections stained with hematoxylin and eosin (H&E) to assess necrosis, inflammation, and fibrosis.
- Cytokine profiling (e.g., ELISA): Measurement of proinflammatory cytokines such as TNF-α, IL-6, and IL-1β to assess inflammatory responses.
- Liver enzyme levels: Serum biomarkers such as ALT, AST, and bilirubin levels to assess liver function and hepatocyte damage.
- Oxidative stress markers: Quantification of reactive oxygen species (ROS), malondialdehyde (MDA), and glutathione levels.
- Gene/protein expression profiling: RT-qPCR and Western blotting techniques to analyze the expression of inflammation-related genes and proteins such as NF-κB, TNF-α, and iNOS.
Additionally, our team can assist in customizing experimental designs and analysis methods to align with specific research objectives, ensuring a tailored approach for every project.
Related Services
In addition to the APAP induced Acute Liver Injury Model, our company offers other models for inducing acute liver injury. These alternative models allow for the study of different mechanisms of liver injury, such as toxin induced damage or immune-mediated liver inflammation. Our team can help select the most suitable model for your research needs.
- CCL4 induced Acute Liver Injury Model
- Concanavalin A (Con A) induced Acute Liver Injury Model
- Polyinosinic: Polycytidylic Acid induced Acute Liver Injury Model
- Alcohol induced Acute Liver Injury Model
- Ischemia-Reperfusion induced Liver Injury Model
- Alpha-Naphthylisothiocyanate (ANIT) induced Acute Liver Injury Model
- DDC (3,5-Diethoxycarbonyl-1,4-Dihydrocollidine) induced Acute Liver Injury Model
Advantages
- Validated Models: We offer well-established and validated APAP induced Acute Liver Injury models, ensuring reliable and reproducible results.
- Tailored Solutions: Our team works closely with clients to customize models and experimental designs to meet specific research goals.
- Comprehensive Services: From model selection to data analysis, we provide full support at every stage of your project, ensuring efficiency and success.
- Cutting-Edge Technologies: We utilize the latest technologies for monitoring liver damage, inflammation, and oxidative stress, providing detailed and accurate data.
- Global Reach: With clients and collaborators worldwide, we bring a broad range of expertise and insights to your research.
- Regulatory Compliance: All models and studies comply with ethical standards and regulatory guidelines, ensuring that your research meets industry requirements.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What is the primary use of the APAP induced Acute Liver Injury model?
This model is primarily used to study drug induced liver injury, mechanisms of hepatotoxicity, and to evaluate potential hepatoprotective drugs.
-
2. What are the advantages of using the APAP induced model?
The APAP model closely mimics human liver injury caused by drug toxicity, offering a reliable platform for studying the mechanisms of liver damage and testing therapeutic interventions.
-
3. Can this model be used to evaluate chronic liver diseases?
The APAP model induces acute liver injury and does not fully replicate chronic liver diseases such as cirrhosis or NAFLD. However, it is widely used for acute toxicity testing.
-
4. What measurements can be used to assess liver injury in this model?
Liver injury can be assessed using a variety of measurements, including liver enzyme levels (ALT, AST), histological analysis, oxidative stress markers, and cytokine profiling.
-
5. Can the APAP model be modified for specific research needs?
Yes, we offer customized solutions and can modify experimental protocols to suit specific research objectives, including changes in dosage, time points, and additional biomarkers.
Published Data
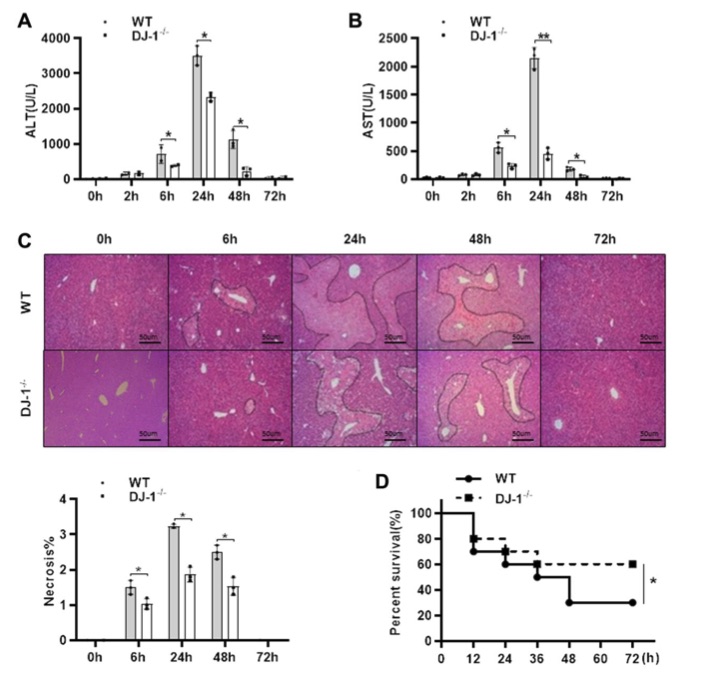 Fig.2 Ablation of DJ-1 protects APAP induced acute liver injury and mortality in mice.2
Fig.2 Ablation of DJ-1 protects APAP induced acute liver injury and mortality in mice.2
To investigate the role of DJ-1 in the pathogenesis of APAP induced liver injury, a classic hepatotoxicity model was utilized. Wild-type (WT) and DJ-1-deficient (DJ-1−/−) mice were fasted and intraperitoneally injected with a single dose of 300 mg/kg of APAP. Compared to WT mice, DJ-1−/− mice exhibited greater resistance to APAP induced hepatotoxicity at various time points, as shown by significantly reduced levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (Figure 2A, 2B) and a smaller necrotic area in liver tissues (Figure 2C) within the first 6 to 48 hours after APAP administration. Additionally, when a higher dose of 500 mg/kg APAP was administered to assess survival rates, DJ-1−/− mice displayed a significantly lower mortality rate than WT mice (Figure 2D). These findings suggest that DJ-1 positively contributes to APAP induced hepatotoxicity, and its deficiency mitigates liver injury and improves survival outcomes.
References
- Li, Xiaoyangzi et al. "Natural Products for Acetaminophen induced Acute Liver Injury: A Review." Molecules (Basel, Switzerland) vol. 28,23 7901. 1 Dec. 2023, DOI:10.3390/molecules28237901. Distributed under an Open Access license CC BY 4.0, without modification.
- Wang, Bingrui et al. "Myeloid DJ-1 deficiency protects acetaminophen induced acute liver injury through decreasing inflammatory response." Aging vol. 13,14 (2021): 18879-18893. DOI:10.18632/aging.203340. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
