Polyinosinic:Polycytidylic Acid (Poly I:C) induced Acute Liver Injury Modeling & Pharmacodynamics Service
Creative Biolabs offers a range of well-established, reliable models for evaluating drug efficacy in the Acute Liver Injury field, enabling precise assessments of therapeutic agents in preclinical research.
Introduction
Acute liver injury (ALI) refers to the rapid onset of liver damage that can lead to liver failure if not managed properly. It is a critical condition that results from various etiologies, such as viral infections, toxic drug overdoses, ischemia-reperfusion injury, and autoimmune diseases. ALI is characterized by hepatocyte necrosis, inflammation, and oxidative stress, often leading to elevated liver enzymes, jaundice, and in severe cases, multi-organ failure. The progression of ALI may vary, but if not treated, it can rapidly evolve into acute liver failure, necessitating liver transplantation in extreme cases. One of the most widely used methods to study ALI is through animal models, which provide insights into the underlying mechanisms of injury and potential therapeutic interventions. Models such as the Polyinosinic-Polycytidylic Acid (Poly I:C) induced ALI model are particularly valuable for studying immune-mediated liver damage, simulating conditions similar to viral infections. These models allow researchers to assess the role of immune responses, inflammation, and cell death in liver injury, providing a platform for evaluating new treatments aimed at liver protection, immune modulation, and antiviral therapies.
Disease Models and Applications
The Polyinosinic-Polycytidylic Acid induced Acute Liver Injury (Poly I:C-ALI) model is an experimental method used to replicate the pathological features of acute liver injury triggered by viral infections. This model is created by injecting Poly I:C into rodents, which activates the innate immune response and induces inflammation, hepatocyte damage, and oxidative stress. Poly I:C mimics the effects of viral infections by stimulating TLR3, leading to cytokine release and immune cell infiltration in the liver. This model is beneficial for evaluating the role of immune responses in liver injury and testing drugs aimed at modulating immune activation. Its advantages include the ability to simulate viral induced liver injury, but its limitations include the lack of direct viral infection and the acute nature of the injury, which may not fully reflect chronic liver diseases. This model serves as an essential tool for assessing therapies targeting immune modulation, antiviral agents, and hepatoprotective compounds.
- Simulates: The Poly I:C induced model simulates acute liver injury triggered by viral infections, including features of inflammation, immune response, and hepatocyte damage.
- Evaluates Drugs: This model is used to evaluate drugs targeting liver inflammation, antiviral agents, immunomodulatory therapies, hepatoprotective agents, and compounds that address oxidative stress or immune cell infiltration.
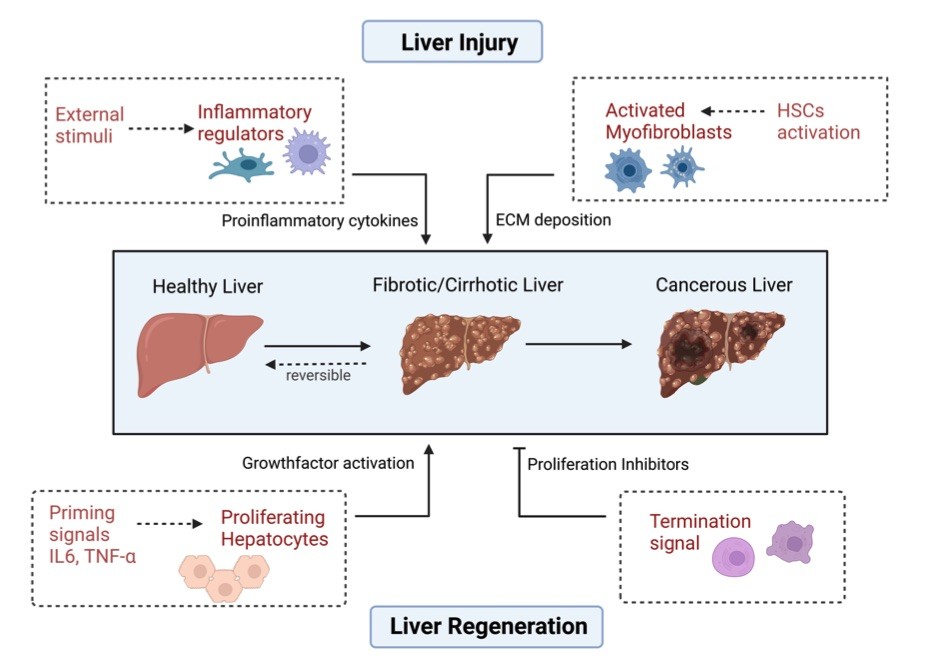 Fig. 1 Various factors influence liver homeostasis.1
Fig. 1 Various factors influence liver homeostasis.1
Measurements
For the Poly I:C induced Acute Liver Injury Model, we offer various measurements to assess drug efficacy, including:
- General observations: Body weight, survival rate, stool consistency, and signs of liver failure.
- Histology and Immunohistochemistry: Examination of liver tissues for immune cell infiltration (e.g., T-cells, macrophages), necrosis, and fibrosis.
- Cytokine profiling (e.g., ELISA): Quantification of inflammatory mediators such as TNF-α, IL-6, and IL-1β.
- Liver Enzyme Analysis: Measurement of serum ALT, AST, and other liver function tests.
- Oxidative Stress Markers: Assessment of reactive oxygen species (ROS) levels, malondialdehyde (MDA), and antioxidant enzymes.
- Gene/Protein Expression Profiling: RT-qPCR and Western blot to analyze the expression of genes and proteins related to inflammation and liver damage (e.g., NF-kB, TNF-α, IL-6).
In addition to these established parameters, our scientific team can assist in customizing experimental design and data analysis to fit your specific needs.
Related Services
In addition to the Poly I:C induced Acute Liver Injury model, our company offers alternative methods to induce acute liver injury. Our flexible approach allows us to choose the most suitable model based on the research focus and therapeutic targets.
- CCL4 induced Acute Liver Injury Model
- Concanavalin A (Con A) induced Acute Liver Injury Model
- Acetaminophen (APAP) induced Acute Liver Injury Model
- Alcohol induced Acute Liver Injury Model
- Ischemia-Reperfusion induced Liver Injury Model
- Alpha-Naphthylisothiocyanate (ANIT) induced Acute Liver Injury Model
- DDC (3,5-Diethoxycarbonyl-1,4-Dihydrocollidine) induced Acute Liver Injury Model
Advantages
- Broad Range of Models: We offer various inducible Acute Liver Injury models, such as Poly I:C and CCl4, to match the specific needs of your research, whether it focuses on immune responses or toxin induced damage.
- High-Quality Data: Our models are designed to yield precise, high-fidelity data for drug evaluation, ensuring that the results of your studies are robust and actionable.
- Tailored Approaches: We work closely with clients to tailor models and experimental protocols, ensuring alignment with specific project goals and therapeutic targets.
- Cutting-Edge Technology: We incorporate advanced technologies for measuring inflammatory markers, oxidative stress, gene expression, and histological analysis, providing a comprehensive view of liver injury mechanisms.
- Regulatory Assurance: Our services comply with global regulatory standards, ensuring that your research aligns with ethical and safety guidelines.
- Global Clientele: Serving researchers worldwide, we provide support and expertise that extends across various therapeutic areas, offering an integrated approach to drug development.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What are the main applications of the Poly I:C induced Acute Liver Injury model?
The model is primarily used to study immune-mediated liver injury and evaluate therapeutic interventions for viral infections and liver inflammation.
-
2. What types of drugs can be tested in this model?
This model is ideal for testing antiviral agents, immune modulators, hepatoprotective compounds, and drugs targeting oxidative stress or inflammation.
-
3. Can this model replicate chronic liver diseases?
The Poly I:C model is suitable for acute liver injury and immune responses, but may not fully represent chronic liver conditions like cirrhosis or NAFLD.
-
4. How do you assess drug efficacy in this model?
Drug efficacy is assessed using a variety of measurements, including histological examination, cytokine profiling, liver enzyme analysis, and gene/protein expression assays.
-
5. Can we customize the model to suit specific research needs?
Yes, our team works closely with you to modify the model and tailor the experimental design to your project requirements.
Published Data
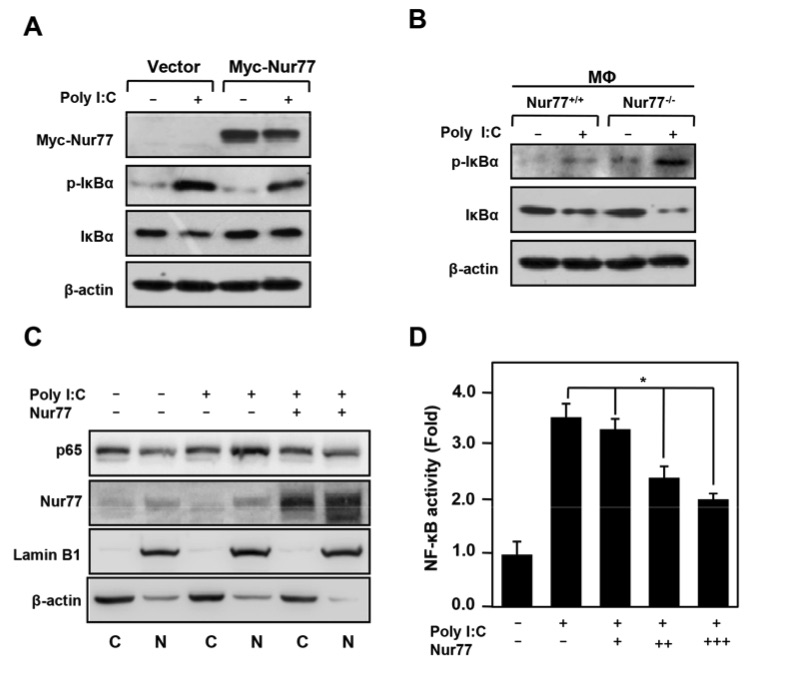 Fig. 2 Nur77 inhibits poly (I:C) induced NF-κB activation.2
Fig. 2 Nur77 inhibits poly (I:C) induced NF-κB activation.2
To investigate whether Nur77 attenuates poly (I:C) induced acute liver inflammation by regulating NF-κB activation, the effects of Nur77 on IκBα phosphorylation and degradation were assessed. Overexpression of Nur77 significantly impaired the poly (I:C) induced phosphorylation and degradation of IκBα (Figure 2A). In contrast, peritoneal macrophages from Nur77-/-mice exhibited increased phosphorylation and degradation of IκBα after poly (I:C) treatment compared to macrophages from Nur77+/+ mice (Figure 2B). Additionally, Nur77 overexpression significantly inhibited the nuclear translocation of p65 induced by poly (I:C) (Figure 2C). Moreover, Nur77 overexpression suppressed NF-κB activation in a dose-dependent manner, further supporting the conclusion that Nur77 negatively regulates the NF-κB inflammatory signaling pathway (Figure 2D). These results indicate that Nur77 plays a critical role in mitigating poly (I:C) induced acute liver inflammation by inhibiting NF-κB activation.
References
- Hora, Shainan, and Torsten Wuestefeld. "Liver Injury and Regeneration: Current Understanding, New Approaches, and Future Perspectives." Cells vol. 12,17 2129. 22 Aug. 2023, DOI:10.3390/cells12172129. Distributed under an Open Access license CC BY 4.0, without modification.
- Li, Xiu-Ming et al. "Orphan nuclear receptor Nur77 inhibits poly (I:C)-triggered acute liver inflammation by inducing the ubiquitin-editing enzyme A20." Oncotarget vol. 8,37 61025-61035. 9 May. 2017, DOI:10.18632/oncotarget.17731. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
