Selection of Cell Type, Environmental Factors & Cell Culture Conditions
Within antibody product characteristics, glycosylation represents one of the most crucial quality attributes and the most difficult parameter to control due to its complex structures and sensitivity to manufacturing processes. Many procedures involved during the cell line development process are significant for the final product glycosylation profile. Clearly known factors that contribute include the host cells, the protein itself, the culture conditions, and various environmental variables. As a professional pioneer in the antibody market, Creative Biolabs has successfully accomplished numerous custom services to illustrate antibody bioactivity and mechanism of action (MOA). In particular, we can provide rational host cell glycoengineering strategies for a desirable selection of cell types, environmental factors, and cell culture conditions.
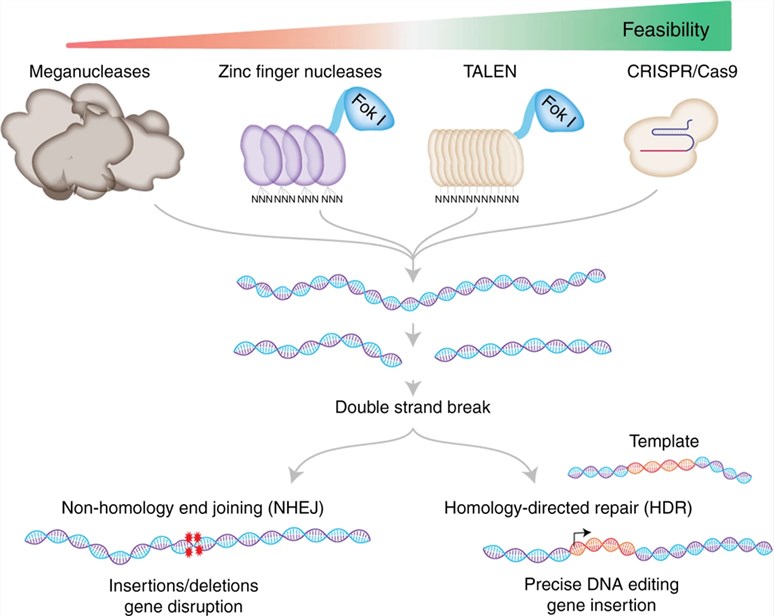 Fig.1 The basic working principle of major genome-editing technologies.1
Fig.1 The basic working principle of major genome-editing technologies.1
Cell Type Development
Glycosylation differs between distinct host cells and even within a population of the same cells. The choice of host cell groups is crucial to determining the glycosylation profile of a protein product. Apart from using vector design and clonal selection to obtain a clone capable of generating drugs with pre-targeted glycosylation patterns, various methods can be performed to engineer the glycosylation machinery of the host cell lines. Alternatively, the glycans of the protein products can be chemoenzymatically remodeled. In Creative Biolabs, glycoengineering of host cell lines could be selected by untargeted screening or targeted gene-genome editing tools.
- Lectin-based glycosylation mutant isolation - An approach utilizes cytotoxic lectins for negative selection of glycosylation mutants and lectins can be selected properly based on the targeted glycosylation feature. This screening-based glycosylation mutant selection using lectins is a common, highly-efficient methodology. For instance, maackia amurensis agglutinin (MAA) is specific for Sia (α2,3) Gal linkage, which is expressed on Chinese hamster ovary (CHO) cells.
- Targeted gene editing tools - This approach is suitable for selecting glycosylation mutants when there’re multiple copies of the same glycogene or functional redundancy. With the advent of gene editing tools, it is possible to create nonlethal knockout by targeting a single or a set of genes for cell engineering.
Environmental Factors
To identify cell lines ideal for the final production, thousands of clones are screened through multiple phases of evaluation. Nowadays, we could choose the most suitable bioreactors to mimic the optimal environments (e.g. temperature, humidity, lighting, and scale) for specific glycoprotein.
Typically, a primary screen is first carried out on a small scale, for example, 24- and 96-well plates for productivity assessment. Then, high-producing clones are identified and scaled up to larger containers, using shake flasks and micro bioreactors. A larger culture volume at this stage offers sufficient material for more detailed characteristics of cell growth, metabolism, titer (volumetric productivity) and other product quality attributes, such as glycosylation. The short-list of top-performing clones will finally be characterized again for comprehensive product quality attributes in laboratory-scale bioreactors with well-controlled culture environments to mimic the final large-scale manufacturing.
During the past years, Creative Biolabs has devoted to improving the efficiency of creating cell lines with high productivity. With optimized expression vectors, high-throughput clonal screening methods and improved media formulation, we can achieve the yield to even more than 2 g/L. In parallel, the timeline to manufacture a high producing cell line has been reduced from over a year to only a few months.
Cell Culture Conditions
Culture conditions are a class of upstream bioprocess parameters that affect glycosylation. To exert control over the predefined glycosylation critical quality attributes, we take efforts to understand the impact of culturing and manufacturing conditions on the glycan distribution in the product. Except for host cell line selection and engineering, the conditions used in running the bioreactor have been displayed to affect the glycan profile on the biotherapies in a product-specific manner. Among the numerous process parameters, we focus on these common conditions that have been studied extensively and their effects on glycosylation.
- Medium (components, attributes)
- Dissolved oxygen tension (DOT)
- pH value/[NH4+]
- Temperature
- Nutrient supplement
- Resistance
At Creative Biolabs, we believe that human compatible and consistent glycosylation is required for a safe and effective antibody product. Monitoring glycosylation has always been a long-standing challenge that asks for in-depth knowledge of glycosylation pathways in cell culture processes. Notably, we’re recognized drug developers in the world that can analyze the glycosylation systematically throughout the antibody development and manufacturing processes. If you’re interested in our services, please contact us for more information.
Reference
-
Adli, Mazhar. "The CRISPR tool kit for genome editing and beyond." Nature Communications 9.1 (2018): 1911.
Distributed under Open Access License CC BY 4.0, without modification.
For Research Use Only.
