TAA Validation
Tumor-associated antigen (TAA) generally refers to an antigenic substance that is newly expressed or overexpressed during tumorigenesis and progression. For example, embryonic antigens, differentiation antigens, and overexpressed oncogene products. TAA is not unique to tumor cells, in normal cells they can be synthesized in a small amount, and are highly expressed in tumor cell proliferation, which can reflect the presence of tumors in vivo to some extent. Remarkably, Creative Biolabs has extensive experience in antigen identification and preclinical antigen validation, we are dedicated to developing TAA validation tools and provide high-quality services to satisfy clients’ needs.
Why Do We Verify TAA?
- It is helpful for the diagnosis of some tumors and offers a basis for therapeutics and drug development.
- It is capable of predicting or monitoring tumor recurrence or metastasis.
- It is useful to help assess the effectiveness of diagnosis, treatment, and prognosis.
TAA Validation Services at Creative Biolabs
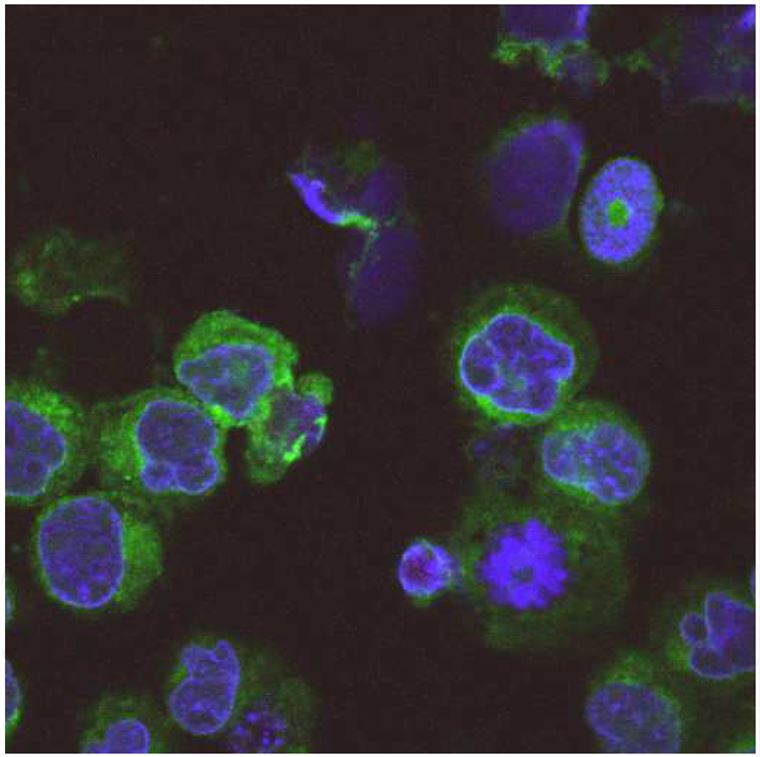 Fig. 1 Demonstration of the sub-cellular localization of the tumor antigen SSX2IP in K562 cells using immunofluorescence microscopy.1
Fig. 1 Demonstration of the sub-cellular localization of the tumor antigen SSX2IP in K562 cells using immunofluorescence microscopy.1
A compromised immune system results in a rising tumor incidence and cancer patient sera evidences the recognition of autologous cancer antigens. The identification of TAAs is critical for us to understand how cancer cells can inhibit the immune system and how the tumor immune suppression can be overcome to break tolerance and achieve cancer destruction.
A number of TAAs have been shown to be useful biomarkers for cancer diagnosis and survival. Once these antigens are identified, their expression in tumor cells requires to be confirmed. At Creative Biolabs, there’re a variety of powerful expression & functional validation services of TAAs that can be provided to perform in tumor cells. Techniques commonly used including but not limited to:
Expression validation
Once TAAs have been identified, their expression in tumors needs to be confirmed. Here, Creative Biolabs holds a number of assays available to validate the expression of antigens in tumor cells, including reverse transcription (RT-PCR)/real-time PCR, enzyme-linked immunosorbent assay (ELISA), immunoblotting (WB), immunoprecipitation, immunocytochemistry/histochemistry, SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF MS) analysis.
Model interaction (computer-aided)
Computer models are a rapid, convenient, and relatively inexpensive option for initial screening of both candidate targets and attractive drugs. The models are often centered on how the two classes of candidate structures interact with each other. Today, Creative Biolabs has had a set of software for this process, covering ligand-based design, virtual screens, and docking programs. If the structure of target protein’s active sites is known, we can predict potential binding sites and evaluate interactions between these sites and drug candidates. After TAAs identified by these computer tools that can model drug-receptor interactions, we also provide state-of-art software to help clients design the chemical probes used in target validation.
Target modulation (sense reversal)
Another route to target validations on inhibiting gene expression to decrease the level of a corresponding protein, and thus identify the physiological role of interested target. Examples for this technique involve antisense technology, gene knockouts, and RNA interference (RNAi). Among them, antisense method is an emerging technique using short oligonucleotides to target specific mRNAs for destruction.
Recently, Creative Biolabs has developed antisense technology to identify oligonucleotide-based drugs that disrupt gene expression, rather than protein function. In particular, this technology is a greater high-throughput tool of target validation as it has a highly efficient and specific ability to prevent the expression of potential target proteins in vivo and in vitro. We’d like to introduce custom target validation packages that contain optimized antisense inhibitors against any targets and control oligonucleotides for testing in cell culture systems. Furthermore, we have applied antisense technology to in vivo target validation in animal models.
In vivo validation (animal model)
The largest challenge in target validation is the research and development of correct animal models for human disease. One of the most popular models is the mouse, but working with mice can be both expensive and time-consuming. As such, Creative Biolabs has settled down this problem by industrializing the generation of mouse knockouts, through gene targeting, gene trapping, and mouse embryonic-stem-cell techniques. There’re many other mammal models you can find in our company. Besides these creatures, one kind of fish, zebrafish have currently entered the fray as an animal model for several disorders. They’re much cheaper, easier to keep, and faster to raise compared with mammals, showing a higher-throughput system.
Immunological validation
TAAs are essentially antigen molecules and most of frequently used validation methods rely on a special antibody. There are several methods that can be used to accomplish immunological validation, such as peptide-specific cytotoxic T lymphocytes (CTL) generation, epitope tagging, epitope identification, tumor cell killing, epitope modification.
Our Highlights
- The most authoritative experimental design and solutions to facilitate customized needs for clients.
- Imported instruments that meet the national quality standards, ensure the specification of experimental operation.
- Accurate TAA validation analytical results through high-quality and -efficiency statistical data.
- Rich domestic and international research background and experience, offering technical support for TAA projects.
As a top-ranking partner in the field of target discovery, Creative Biolabs always devotes to examining TAA’s role as biomarkers for treatment response, patient survival, and personalized therapies. Our experts have established TAA identification and characterization platforms based on the most advanced equipment and technologies. We’re skilled at exploiting antigen recognition technologies for clinical trials and are pleased to offer expression and functional validation of TAA targets by human-relevant disease systems. Aided by us, clients can achieve the efficacy assessment of TAAs in cancer cells and healthy cells, harvest patient-specific stem cell generation, and produce customized versions of stem cells-derived cell types as requests. If you want to know more information about TAA validation, please don’t hesitate to contact us.
Reference
- Khan, G.; et al. Identification and validation of targets for cancer immunotherapy: from the bench-to-bedside. licensee InTech. 2013, 12: 1-31. Distributed under Open Access license CC BY 3.0, without modification.
For Research Use Only.
