High-Fat & High-Cholesterol (HFHC) Diet induced Heart Failure Modeling & Pharmacodynamics Service
Creative Biolabs is dedicated to advancing this understanding by providing a variety of well-established rodent models to rigorously evaluate the efficacy of novel HF interventions.
Introduction
Heart failure (HF) represents a complex and debilitating clinical syndrome where the heart is unable to pump sufficient blood to meet the body's metabolic demands. This progressive condition impacts millions globally, often stemming from underlying cardiovascular risk factors. Understanding its intricate mechanisms and identifying effective therapies are paramount for improving patient outcomes.
High-Fat and High-Cholesterol Diet-Induced HF Model
The modern dietary landscape, rich in saturated fats and cholesterol, significantly contributes to the escalating prevalence of metabolic syndrome and, subsequently, HF. Our high-fat and high-cholesterol (HFC) diet-induced HF models are meticulously designed to emulate key aspects of human disease progression, offering a robust platform for preclinical investigation.
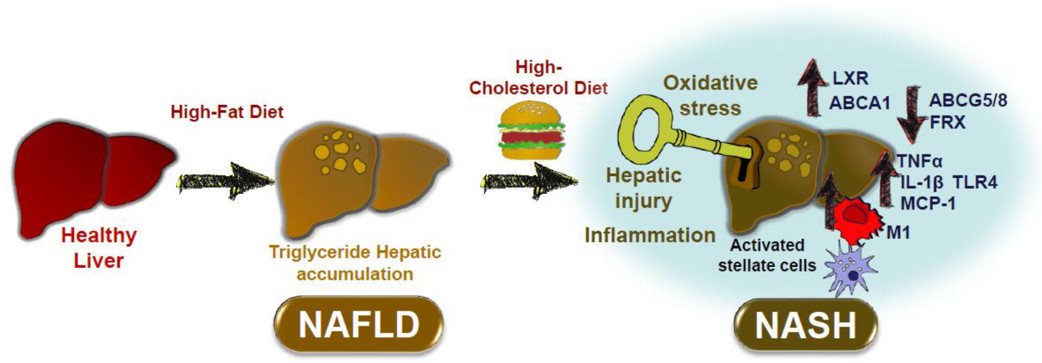 Fig.1 Effect of HFC diet on the progression of non-alcoholic steatohepatitis (NASH).1,3
Fig.1 Effect of HFC diet on the progression of non-alcoholic steatohepatitis (NASH).1,3
Model Construction Steps
Model construction involves a controlled, long-term dietary regimen designed to induce progressive metabolic and cardiac dysfunction in susceptible rodent strains.
01Strain Selection
We select appropriate rodent strains based on their susceptibility to diet-induced pathologies, such as male C57BL/6 mice for high-fat diet response, or SHRSP5/Dmcr rats for predisposition to hypertension and NASH.
02Diet Formulation
Specialized HFC diets are formulated; for instance, C57BL/6 mice often receive 60% fat-derived calories (e.g., D12412), while SHRSP5/Dmcr rats get a specific HFC blend including palm oil, cholesterol, and cholic acid to induce NASH and cardiac changes.
03Feeding Regimen
Rodents are maintained on the HFC diet for an extended, critical period, typically 8 to over 24 weeks, ensuring full pathological emergence. Concurrent control groups, fed a standard or low-fat diet (e.g., 10% calories from fat), provide baseline comparison.
04Phenotype Development
HFC diet-fed animals progressively develop key metabolic syndrome features—obesity, dyslipidemia, insulin resistance, systemic inflammation, and oxidative stress—ultimately driving cardiac hypertrophy, myocardial fibrosis, and left ventricular diastolic dysfunction, mirroring human HF.
Strengths and Limitations
Strengths:
- High Translational Relevance: These models closely mimic human diet-induced metabolic syndrome and its progression to HF, offering strong translational value for therapeutic discovery.
- Comprehensive Pathology: They recapitulate a broad range of cardiac and systemic pathologies, including cardiac hypertrophy, fibrosis, vascular dysfunction, and often NASH.
- Reproducibility: When established with meticulous protocols, these models provide consistent and reliable phenotypic outcomes.
- Utility for Prevention and Intervention: Ideal for evaluating both preventive strategies and interventions aimed at reversing established disease.
- Diverse Strain Options: Availability of various susceptible rodent strains allows for model selection based on specific research questions (e.g., C57BL/6 for obesity-related cardiomyopathy, SHRSP5/Dmcr for NASH-cardiac axis).
Limitations:
- Time-Intensive: Inducing robust cardiac pathology typically requires prolonged feeding periods, making studies longer compared to acute injury models.
- Variability: While generally reproducible, individual variability in response to diet can occur, necessitating adequate group sizes.
- Diet Complexity: Precise diet composition and consistency are crucial to ensure successful model induction.
Evaluation Platform
Our comprehensive evaluation platform allows for in-depth characterization of the HFC diet-induced HF model and the precise assessment of therapeutic efficacy. We employ a multi-faceted approach combining cutting-edge instruments and validated tests across various biological levels:
- Biochemical: Lipid panel (TC, TG, LDL-C, HDL-C), glucose, insulin, inflammatory markers (e.g., CRP, IL-6, TNF-α), oxidative stress markers (e.g., MDA, SOD activity), cardiac injury biomarkers (e.g., troponin I, H-FABP).
- Molecular: Gene expression (RT-qPCR, RNA-seq) and protein expression (Western blot, ELISA) of targets related to cardiac remodeling, inflammation, metabolism, and oxidative stress.
- Cellular: Isolation and assessment of cardiomyocyte contractility (myocardial mechanics), cellular viability, and apoptosis.
- Histopathological: Staining techniques (e.g., Masson's Trichrome for fibrosis, Oil Red O for lipid accumulation) to evaluate cardiac hypertrophy, fibrosis, and steatosis in heart tissue.
- Imaging: Advanced echocardiography (LV dimensions, wall thickness, LVEF, E/A ratio, E/e' ratio for diastolic function) and potentially micro-CT or MRI for comprehensive structural and functional assessment.
- Physiological/Behavioral: Body weight, fat mass, glucose tolerance tests (GTT), insulin sensitivity tests (ITT), blood pressure measurements, and invasive hemodynamics (pressure-volume loops) for precise cardiac function.
Applications
- Disease Modeling: These models accurately simulate complex human cardiovascular conditions such as obesity-related cardiomyopathy, HFpEF, diabetic cardiomyopathy, NASH-associated CVD, and atherosclerosis, providing a relevant platform for studying disease pathogenesis.
- Therapeutic Efficacy Testing: They are instrumental for evaluating diverse pharmacological interventions, including anti-obesity, lipid-lowering, anti-diabetic, anti-inflammatory, and anti-fibrotic drugs, as well as novel compounds targeting cardiac remodeling or metabolic pathways.
- Broad Treatment Evaluation: The models also support the assessment of various treatment modalities beyond traditional pharmaceuticals, encompassing nutraceuticals, dietary supplements, lifestyle interventions like exercise, and combination therapies, to address the multi-factorial nature of HF.
Related Heart Failure Models
PA Constriction induced Right HF Model
Ascending Aortic Arch Constriction induced Post-Pressure Overload Heart Failure Model
Abdominal Aortic Stenosis induced Left HF Model
DOCA & Salt induced Left HF Model
AngII induced Chronic Heart Failure Model
ACF induced Anterior Pressure Overload Heart Failure Model
HFD induced Heart Failure Model
5/6 Nephrectomy induced Heart Failure Model
Pulmonary Hypertension induced Right Heart Failure Model
Renal Artery Constriction induced Hypertensive Heart Failure Model
Our Advantages
- Validated Models: Meticulously validated HFC diet-induced HF models, ensuring high translational relevance.
- Reliable Data Generation: Commitment to producing dependable and consistent research outcomes.
- Comprehensive Phenotyping: Extensive capabilities from biochemical analysis to advanced imaging, providing unparalleled insights.
- Tailored Study Designs: Flexible and customized protocols precisely aligned with your unique research objectives.
- Accelerated Discovery: Streamlined processes to expedite your journey from preclinical findings to clinical application.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
Contact Us
Creative Biolabs is dedicated to providing high-quality preclinical research services that propel cardiovascular drug discovery forward. We invite you to contact us to discuss how our HFC Diet-Induced HF Models can be integrated into your next research program and contribute to your success.
FAQs
-
Q1: What are the key differences between various high-fat diet models, and which is most suitable for my research?
A: HFC diet models vary by fat content, source, and rodent strain, yielding distinct pathological outcomes. For example, C57BL/6 mice on 60% fat diets often exhibit obesity, insulin resistance, and diastolic dysfunction, while specific HFC diets in SHRSP5/Dmcr rats more readily induce NASH alongside cardiac fibrosis and vascular dysfunction. Selecting the ideal model for your HF research requires expert guidance based on your therapeutic targets and goals.
-
Q2: Are your HFC diet models suitable for evaluating both preventive and therapeutic interventions?
A: Absolutely. Our HFC models are versatile for both preventive and interventional studies. Preventive studies involve co-administering test compounds with the HFC diet from the outset to evaluate disease mitigation. For therapeutic interventions, compounds are introduced after phenotype establishment, allowing assessment of their capacity to halt or reverse disease progression.
-
Q3: What is your capacity for large-scale studies involving these models for drug screening?
A: Creative Biolabs possesses substantial capacity for large-scale drug screening studies. Our state-of-the-art facilities, experienced staff, and streamlined protocols allow simultaneous management of multiple cohorts. We are equipped for high-throughput screening, ensuring efficient progression of your drug discovery pipeline and robust data for lead optimization.
-
Q4: How do you ensure the ethical treatment and welfare of animals in these long-term diet studies?
A: Ethical animal welfare is paramount at Creative Biolabs. All studies strictly adhere to the highest international animal care and use guidelines. Our accredited facilities ensure rigorous review and approval by an IACUC. Animals are continuously monitored, and humane endpoints are established, ensuring their welfare throughout long-term dietary interventions.
-
Q5: Can you customize the diet composition or duration based on specific research needs?
A: Yes, customization is a core strength. We recognize unique research requirements and can tailor diet composition (e.g., varying fat percentages, cholesterol levels, or fat sources) and adjust feeding duration. Our scientific team collaborates closely to design study protocols that perfectly address your specific hypotheses and objectives.
Published Data
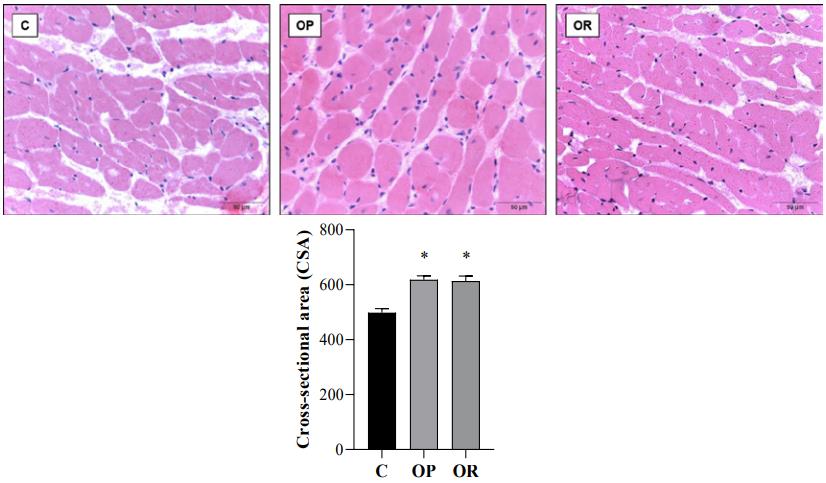 Fig.2 Cross-sectional area (CSA) determination of left ventricular fragments.2,3
Fig.2 Cross-sectional area (CSA) determination of left ventricular fragments.2,3
This study demonstrated how a high-fat diet induced cardiac damage in obesity-resistant rodents, alongside a reduction in metabolic health. This research, conducted in a context where obesity was not the primary driver, highlighted the direct cardiotoxic effects of the high-fat diet, revealing increased cardiac mass, left ventricular CSA, and elevated cholesterol levels compared to control groups. This open-access publication provides valuable insights into diverse manifestations of diet-induced cardiac injury.
References
- Vinué, Ángela et al. "Understanding the Impact of Dietary Cholesterol on Chronic Metabolic Diseases through Studies in Rodent Models." Nutrients vol. 10,7 939. 21 Jul. 2018. https://doi.org/10.3390/nu10070939
- Cardoso, Janete Corrêa et al. "A High-Fat Diet Induces Cardiac Damage in Obesity-Resistant Rodents with Reduction in Metabolic Health." Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology vol. 57,4 (2023): 264-278. DOI: 10.33594/000000642
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
