CD4⁺CD45RBhi T Cell induced Inflammatory Bowel Disease (IBD) Modeling & Pharmacodynamics Service
Introduction
Inflammatory Bowel Disease (IBD) refers to a group of chronic, relapsing-remitting inflammatory conditions that affect the gastrointestinal tract, with ulcerative colitis (UC) and Crohn's disease (CD) being the two primary forms. Ulcerative colitis is confined to the colon and rectum, causing continuous mucosal inflammation, while Crohn's disease can affect any part of the gastrointestinal tract and is characterized by transmural and segmental inflammation. Both conditions lead to symptoms such as abdominal pain, diarrhea, rectal bleeding, fatigue, and weight loss, significantly impairing patients' quality of life. The pathogenesis of IBD involves a complex interaction among genetic susceptibility, environmental triggers, gut microbiota imbalance, and inappropriate immune responses. Over 200 genetic loci have been associated with IBD, many of which influence immune regulation and epithelial barrier function. Dysregulated cytokine production, including elevated TNF-α, IL-6, and IL-1β, contributes to sustained inflammation and tissue damage. Despite numerous therapeutic options, including corticosteroids, immunosuppressants, biologics (e.g., anti-TNF agents), and small molecule inhibitors, many patients experience treatment failure or relapse, highlighting the need for novel therapeutic strategies. Creative Biolabs offers a full range of rodent IBD models, including DSS-, TNBS-, oxazolone-, acetic acid-, anti-CD40-, and IL-10 knockout models, as well as adoptive T cell transfer models. Each model mimics different disease mechanisms of IBD. Our services cover model establishment, drug dosing, clinical scoring, histopathology, cytokine analysis, and gene expression profiling—providing comprehensive preclinical data to support your IBD drug development pipeline.
Disease Models and Applications
The CD4+CD45RBhi T cell-induced inflammatory bowel disease (IBD) model is a well-established and highly reproducible murine model that mimics chronic colitis driven by T cell-mediated immune responses. This model is typically generated by adoptively transferring naïve CD4+CD45RBhi T cells, isolated from healthy donor mice, into immunodeficient recipients such as Rag1-/-or SCID mice, which lack functional T and B cells. Within 4–8 weeks post-transfer, recipients develop progressive colitis characterized by weight loss, diarrhea, epithelial damage, mucosal infiltration of inflammatory cells, and elevated levels of Th1- and Th17-associated cytokines. The model recapitulates many immunopathological features of human Crohn's disease, including chronic inflammation, loss of tolerance to gut microbiota, and lymphocyte-driven mucosal injury. It is ideal for studying the role of T cells, cytokine signaling, and regulatory mechanisms in IBD. Advantages include its immune specificity, chronicity, and responsiveness to immunomodulatory treatments. However, the model is technically demanding due to cell sorting and transfer procedures, and disease development may vary depending on donor cell purity and host condition.
- Simulates: The CD4+CD45RBhi T cells-induced inflammatory bowel disease (IBD) model simulates chronic, immune-mediated colitis that closely resembles human Crohn’s disease. It replicates key features such as progressive weight loss, mucosal inflammation, epithelial damage, and dysregulated Th1/Th17 responses driven by naïve T cell activation in the absence of regulatory T cells.
- Evaluates Drugs: This model is commonly used to evaluate immunomodulatory therapies targeting T cell activation, differentiation, and cytokine signaling. It is suitable for testing anti-inflammatory agents, biologics (e.g., anti-TNF-α, anti-IL-12/23), JAK inhibitors, regulatory T cell therapies, and novel interventions aiming to restore mucosal immune homeostasis.
Measurements
We offer a variety of measurements for evaluating drug efficacy in the CD4+CD45RBhi T cells-induced inflammatory bowel disease (IBD) model, utilizing advanced and validated techniques, including but not limited to:
- General observations: Body weight monitoring, diarrhea scoring, fecal blood detection, survival rate, and disease activity index (DAI) to assess clinical progression.
- Histopathological analysis: Hematoxylin and eosin (H&E) staining of colon tissue to evaluate mucosal architecture, epithelial injury, crypt loss, ulceration, and inflammatory cell infiltration.
- Cytokine profiling (e.g., ELISA): Quantification of pro-inflammatory cytokines such as IFN-γ, TNF-α, IL-6, IL-17, and IL-1β in colon tissue or serum.
- Flow cytometry: Assessment of immune cell populations (e.g., CD4+ T cells, regulatory T cells, Th1/Th17 subsets) in lamina propria, spleen, and mesenteric lymph nodes.
- Myeloperoxidase (MPO) activity assay: Measurement of neutrophil infiltration as an index of acute inflammation.
- Gene/protein expression profiling: RT-qPCR and Western blot analysis of inflammatory mediators, tight junction proteins, and immune signaling molecules in colon tissue.
Our expertise extends beyond standard models, enabling the development of customized protocols that fit specific therapeutic mechanisms. Our scientific team offers comprehensive support, from T cell isolation and transfer to data interpretation, ensuring high-quality, reproducible results that drive your IBD research forward.
Related Services
In addition to the CD4+CD45RBhi T cell-induced model, we offer a variety of other colitis models, each designed to meet specific research needs. Our team provides expert support to ensure tailored, high-quality results.
- TNBS/DNBS induced Colitis Model
- DSS induced Colitis Model
- Indomethacin induced Small Intestinal Inflammatory Model
- OXA induced Colitis Model
- Acetic Acid induced IBD Model
- Anti-CD40 Ab induced IBD Model
- IL-10 KO Mouse Spontaneous IBD Model
Advantages
- Customization & Flexibility: Our team specializes in tailoring models, dosing regimens, and evaluation parameters to fit specific research goals and drug modalities.
- Advanced Analytical Capabilities: We apply state-of-the-art technologies such as ELISA, RT-qPCR, flow cytometry, Western blotting, and histopathology for detailed mechanistic and efficacy assessments.
- Experienced Scientific Team: Our scientists bring extensive expertise in preclinical inflammation and immunology, offering full support from experimental design to data interpretation.
- Reliable & Reproducible Data: Standardized protocols, quality control measures, and transparent reporting ensure dependable, publication-ready results.
- Efficient Project Delivery: We emphasize responsive communication, milestone tracking, and efficient workflows to accelerate your research timelines without compromising quality.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q1: What types of inflammatory bowel disease (IBD) models do you offer?
A1: We offer a wide variety of IBD models, including DSS-, TNBS-, DNBS-, oxazolone-, acetic acid-, anti-CD40 antibody-induced models, IL-10 knockout mice, and the CD4+CD45RBhi T cell transfer model. Each model captures different immune and pathological aspects of IBD.
-
Q2: Can you help choose the most suitable model for my study?
A2: Yes. Our expert team will work with you to understand your research objectives and recommend the most appropriate model based on disease mechanism, target pathway, and drug characteristics.
-
Q3: What endpoints and analyses are available?
A3: We provide a full range of assessments, including clinical observations (weight, stool, bleeding), colon length, histopathology, cytokine profiling (ELISA), immune cell analysis (IHC/flow cytometry), and gene/protein expression (RT-qPCR, Western blot).
-
Q4: Do you support custom experimental designs?
A4: Absolutely. We offer flexible study designs, custom dosing regimens, time points, and evaluation endpoints tailored to your specific needs.
-
Q5: How do you ensure data quality and reproducibility?
A5: All studies follow validated protocols under strict quality control. We use standardized procedures and include proper controls to ensure consistency and reproducibility across experiments.
Published Data
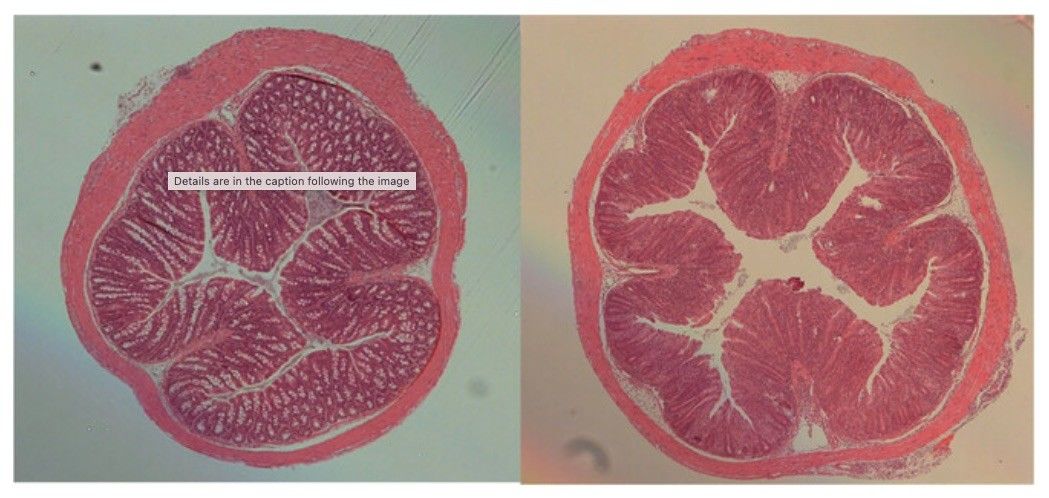 Fig. 1 Microscopic analysis of bowel wall in mouse T cell transfer–induced colitis.1
Fig. 1 Microscopic analysis of bowel wall in mouse T cell transfer–induced colitis.1
This experiment involves the adoptive transfer of naïve CD4+CD45RBhi T cells into immunodeficient Rag⁻/⁻ mice to induce colitis, with or without co-transfer of regulatory T cells. In mice receiving only CD4+CD45RBhi T cells, microscopic analysis shows marked inflammation and epithelial hyperplasia extending throughout the full thickness of the colon wall, with lesions diffusely distributed from the cecum to the rectum (Fig. 1). In contrast, Rag⁻/⁻ mice co-transferred with CD4+CD45RBhi T cells and CD4+CD25+CD45RBlo regulatory T cells exhibit minimal to no pathological changes. Their colonic tissues appear either normal or comparable to those of healthy C57BL/6J controls (Fig. 1), demonstrating the protective role of Treg cells in preventing colitis development.
Reference
- Pearson, Claire F, and Kevin J Maloy. "Update: Induction of Inflammatory Bowel Disease in Immunodeficient Mice by Injection of Naïve CD4+ T cells (T Cell Transfer Model of Colitis)." Current Protocols vol. 4,7 (2024): e1092. doi:10.1002/cpz1.1092. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
