Hypoxia induced Pulmonary Hypertension Modeling & Pharmacodynamics Service
Introduction
Hypertension, or high blood pressure, is a pervasive global health challenge, significantly contributing to cardiovascular morbidity and mortality. While systemic hypertension is widely recognized, pulmonary hypertension (PH) specifically affects the arteries in the lungs and the right side of the heart, leading to severe complications. Understanding and addressing the various forms of hypertension is crucial for developing effective therapeutic strategies.
At Creative Biolabs, we leverage our two decades of expertise to provide a comprehensive suite of well-established and rigorously validated animal models, enabling precise evaluation of novel compounds for various hypertension indications.
Hypoxia-Induced PH Model
The hypoxia-induced pulmonary hypertension (HPH) model is a cornerstone in pre-clinical research for understanding and treating PH, particularly forms associated with chronic low oxygen conditions. This model faithfully recapitulates key pathological features seen in human HPH, including progressive pulmonary vascular remodeling, increased pulmonary arterial pressure, and subsequent right ventricular hypertrophy.
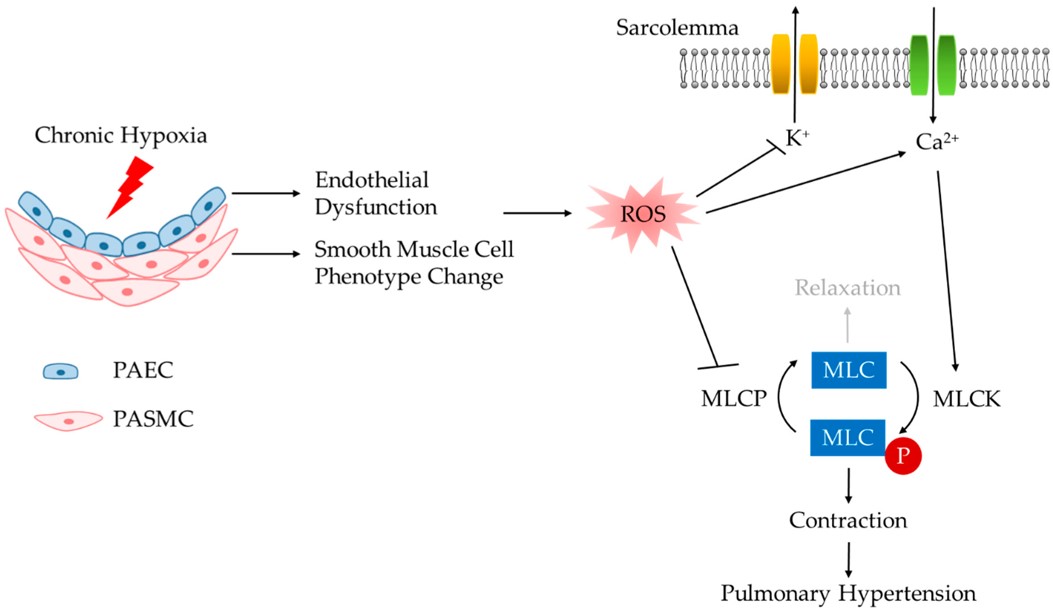 Fig.1 Mechanism of chronic hypoxia-induced PH.1,3
Fig.1 Mechanism of chronic hypoxia-induced PH.1,3
Model Construction Steps
The construction of the HPH model typically involves exposing experimental animals, most commonly rodents, to a precisely controlled chronic hypoxic environment. This sustained low oxygen exposure drives the characteristic pathological changes.
01Animal Selection
Healthy, age-matched rodents are chosen to ensure experimental consistency.
02Hypoxic Exposure
Animals are housed in specialized hypoxia chambers, where oxygen levels are meticulously maintained at a low percentage (e.g., 10% O2) for an extended duration, typically 3-4 weeks. Control animals are housed under normoxic conditions.
03Environmental Control
The hypoxia chambers are equipped with advanced systems to precisely regulate oxygen concentration, temperature, humidity, and CO2 levels, ensuring animal welfare and minimizing environmental variables.
04Optional Augmentation (Hypoxia/SU5416 Model)
For studies requiring a more severe and irreversible form of PH, the hypoxia exposure can be combined with administration of a VEGF receptor antagonist like SU5416. This combination exacerbates vascular remodeling and more closely mimics severe forms of human PAH.
05Monitoring
Animals are regularly monitored for general health, weight, and signs of distress throughout the exposure period.
06Endpoint Collection
At the conclusion of the hypoxic exposure, comprehensive phenotypic characterization is performed to assess the extent of PH development.
Strengths and Limitations
Strengths:
- High Reproducibility: The controlled hypoxic environment ensures consistent and reliable disease induction.
- Clinical Relevance: Recapitulates key pathological features observed in human HPH, offering strong translatability.
- Cost-Effective: Compared to larger animal models, rodent HPH models offer a more economical approach for initial screening and mechanistic studies.
- Established Endpoints: Well-defined and quantifiable endpoints allow for clear assessment of disease progression and therapeutic impact.
Limitations:
- Species Differences: Rodent physiology may not perfectly replicate all aspects of human PH.
- Disease Complexity: While robust, the model may not fully capture the multifactorial etiology of all human PH subtypes.
- Reversibility: The basic chronic hypoxia model can show some reversibility if hypoxia is removed, which might not fully reflect the progressive nature of advanced human PH. The Hypoxia/SU5416 model addresses this to some extent.
Evaluation Platform
Creative Biolabs offers a robust evaluation platform for comprehensive assessment of the HPH model. Our state-of-the-art facilities and experienced team enable detailed biochemical, molecular, cellular, histopathological, and imaging analyses, providing a holistic view of disease progression and therapeutic efficacy.
Key Test Indicators:
- Hemodynamic: Right Ventricular Systolic Pressure (RVSP), Mean Pulmonary Arterial Pressure (mPAP), Pulmonary Vascular Resistance (PVR).
- Cardiac Function: Right Ventricular Hypertrophy (RVH) assessed by Fulton Index (RV/(LV+S)), echocardiography for cardiac output and ejection fraction.
- Histopathology: Pulmonary arterial wall thickness, muscularization, lumen occlusion, collagen deposition (Masson's Trichrome), perivascular inflammation.
- Molecular/Cellular: Gene expression (qPCR), protein expression (Western blot, immunohistochemistry/immunofluorescence) of markers related to inflammation, fibrosis, proliferation, apoptosis, and endothelial function (e.g., α-SMA, PCNA, collagen I/III, ET-1, eNOS).
- Multi-Omics: Advanced capabilities for transcriptomics (e.g., RNA-seq), proteomics, and metabolomics to uncover deeper mechanistic insights.
Applications
- Simulated Diseases: Primarily simulates Group 3 PH (PH due to lung disease and/or hypoxia), including conditions like high-altitude PH, PH associated with Chronic Obstructive Pulmonary Disease (COPD), and sleep apnea. It also provides insights into general pulmonary vascular remodeling.
- Evaluated Drugs: Used to test novel compounds targeting various aspects of PH, such as vasodilators, anti-proliferative agents, anti-fibrotic drugs, anti-inflammatory compounds, and drugs affecting endothelial function.
- Treatment Strategies: Essential for evaluating the efficacy of single-agent therapies, combination therapies, and novel therapeutic approaches (e.g., gene therapy, cell-based therapies, dietary interventions) aimed at preventing or reversing PH progression.
- Biomarker Discovery: Facilitates the identification and validation of novel circulating or tissue-based biomarkers for early diagnosis, disease stratification, and monitoring treatment response.
Related Hypertension Models
Our Advantages
- Decades of Expertise: Years specializing in pre-clinical research with proven success in cardiovascular models.
- Optimized Protocols: Rigorously standardized and validated HPH induction protocols ensure high reproducibility and robust data.
- Comprehensive Characterization: Access to a full spectrum of state-of-the-art phenotypic and molecular analyses.
- Flexible Study Design: Tailored experimental designs to precisely meet your unique research objectives and budget.
- Integrated Solutions: Ability to combine HPH studies with advanced multi-omics analyses for deeper mechanistic understanding.
- Dedicated Scientific Support: Our expert team provides insightful data interpretation and strategic guidance throughout your project.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
Contact Us
Creative Biolabs stands as your premier partner in advancing PH research. We are committed to delivering high-quality, translatable data through our well-characterized HPH model services. Contact us today to discuss your specific research needs and explore how our expertise can accelerate your therapeutic development.
FAQs
-
Q1: What are the key differences between the standard chronic hypoxia model and the hypoxia/SU5416 model for PH?
A: The standard chronic hypoxia model induces a milder form of PH, primarily characterized by vasoconstriction and reversible vascular remodeling. In contrast, the hypoxia/SU5416 model, by adding a VEGF receptor antagonist, leads to more severe and often irreversible PH, featuring pronounced vascular remodeling, including neointimal lesions, which more closely mimics the severe pathology observed in human pulmonary arterial hypertension.
-
Q2: How long does the hypoxic exposure typically last in your HPH model studies?
A: The duration of hypoxic exposure can vary depending on the research objectives and the desired severity of PH. For standard HPH induction, exposure typically ranges from three to four weeks. However, we can customize the duration based on your specific study design, whether you aim for early-stage mechanistic insights or more advanced, chronic disease progression.
-
Q3: Can you help with customized study designs or specific animal strains for HPH research?
A: Absolutely. Our team excels in developing bespoke study designs tailored to your unique research questions. We routinely work with various rodent strains and can accommodate specific genetic backgrounds or modifications. Our flexibility ensures that your experimental setup is optimized to yield the most relevant and impactful data for your therapeutic candidates.
-
Q4: How do you ensure the reproducibility and quality of your HPH model data?
A: Data reproducibility and quality are paramount at Creative Biolabs. We achieve this through stringent adherence to standardized operating procedures, meticulous control of the hypoxic environment, rigorous quality control checks at every stage of the experiment, and the expertise of our highly trained scientific team. All equipment is regularly calibrated, and data analysis is performed with precision.
-
Q5: Is it possible to evaluate the reversibility of pH in your models after therapeutic intervention?
A: Yes, evaluating the reversibility of PH is a common and crucial aspect of drug development. Our HPH models can be designed to assess the regression of established PH following therapeutic intervention. This typically involves treating animals after PH has been induced and then re-evaluating hemodynamic, histological, and molecular parameters to quantify the extent of disease reversal.
-
Q6: Can your HPH model be used to identify novel biomarkers for PH?
A: Indeed. The HPH model is an excellent platform for biomarker discovery. By collecting various biological samples (e.g., plasma, lung tissue) at different stages of disease progression and after therapeutic intervention, we can perform comprehensive molecular profiling to identify and validate novel circulating or tissue-based biomarkers that correlate with disease severity or treatment response.
Published Data
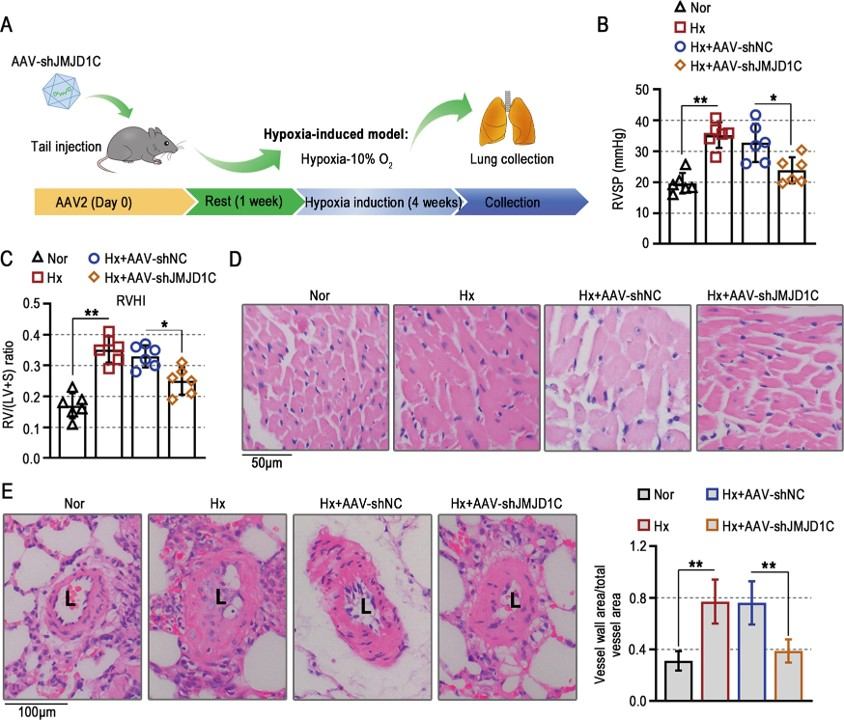 Fig.2 Effect of JMJD1C knockdown on hypoxia-induced PAH.2,3
Fig.2 Effect of JMJD1C knockdown on hypoxia-induced PAH.2,3
This study utilized a hypoxia-induced mouse model of PH to investigate the role of JMJD1C. The project results showed that JMJD1C knockdown significantly ameliorated hypoxia-induced right ventricular remodeling and thickening of the pulmonary arterial wall. Furthermore, JMJD1C inhibition suppressed pulmonary arterial smooth muscle cell hyperproliferation and resistance to apoptosis by reducing glycolytic enzymes and lactate accumulation, suggesting JMJD1C as a novel molecular target for developing new therapies for PAH by regulating metabolic programs and vascular remodeling.
References
- Yan, Simin et al. "Vasoconstrictor Mechanisms in Chronic Hypoxia-Induced Pulmonary Hypertension: Role of Oxidant Signaling." Antioxidants (Basel, Switzerland) vol. 9,10 999. 15 Oct. 2020. https://doi.org/10.3390/antiox9100999
- Zhang, Chen et al. "JMJD1C promotes smooth muscle cell proliferation by activating glycolysis in pulmonary arterial hypertension." Cell death discovery vol. 9,1 98. 18 Mar. 2023. https://doi.org/10.1038/s41420-023-01390-5
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
