Acute Pancreatitis Modeling & Pharmacodynamics Services
Introduction
Acute pancreatitis (AP) is an inflammatory disorder of the pancreas that can vary in severity, ranging from mild to life-threatening conditions. It is most commonly caused by gallstones, excessive alcohol consumption, and certain medications. In severe cases, acute pancreatitis can lead to systemic complications such as organ failure and infection, requiring intensive care and surgical intervention. The condition arises when digestive enzymes become prematurely activated within the pancreas, resulting in inflammation and tissue damage. Patients typically present with severe abdominal pain, nausea, and vomiting, with diagnosis confirmed through blood tests showing elevated amylase and lipase levels, alongside imaging studies. Creative Biolabs provides comprehensive preclinical services to assess drug efficacy in treating acute pancreatitis. We offer various established animal models to evaluate potential therapies, track disease progression, and ensure the reliability of treatment outcomes in a controlled research environment.
Disease Models and Applications
Creative Biolabs offers a comprehensive range of well-established rodent models for acute pancreatitis research, designed to replicate the human condition of acute pancreatitis induced by factors such as sodium taurocholate, caerulein, or a combination of caerulein and LPS administration. These models are carefully developed to simulate the pathophysiology of acute pancreatitis, providing a reliable platform for evaluating potential therapeutic candidates. Our service includes detailed assessments of pancreatic inflammation, necrosis, edema, and serum enzyme levels, offering valuable insights into drug efficacy. Our experienced team will support you throughout the project, from experimental design to data analysis, ensuring accurate and dependable results. To learn more about our acute pancreatitis models for preclinical research, please refer to the links below:
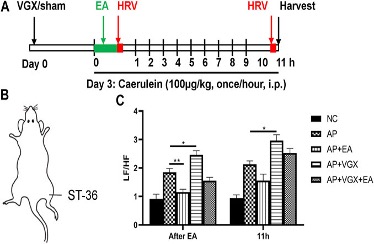 Fig. 1 Effects of electroacupuncture on HRV in caerulein AP mice.1
Fig. 1 Effects of electroacupuncture on HRV in caerulein AP mice.1
-
Sodium Taurocholate induced Acute Pancreatitis Model
- Simulates: This model replicates acute pancreatitis induced by sodium taurocholate, a bile acid that, when administered into the pancreatic duct, causes pancreatic inflammation and tissue damage through bile acid-induced injury and activation of digestive enzymes.
- Drug Evaluation: This model is used to evaluate drugs that target bile acid toxicity, pancreatic inflammation, and enzyme activity. Drugs such as anti-inflammatory agents, enzyme inhibitors, antioxidants, and bile acid sequestrants can be tested for their ability to reduce pancreatic edema, alleviate inflammation, and protect against tissue damage in this model.
-
Cerulein induced Acute Pancreatitis Model
- Simulates: This model mimics acute pancreatitis induced by caerulein, a peptide that stimulates pancreatic enzyme secretion and induces pancreatic edema, inflammation, and necrosis. The repeated caerulein injections lead to a robust inflammatory response in the pancreas, simulating the condition of acute pancreatitis.
- Drug Evaluation: This model is ideal for testing anti-inflammatory drugs, protease inhibitors, cytokine modulators, and pancreatic enzyme modulators. It helps assess drugs that aim to reduce pancreatic inflammation, promote tissue regeneration, and protect pancreatic acinar cells from damage.
-
Caerulein & LPS induced Acute Pancreatitis Model
- Simulates: This model induces severe acute pancreatitis by combining caerulein and lipopolysaccharide (LPS), leading to a more complicated and severe form of inflammation. LPS, a bacterial endotoxin, triggers systemic inflammation and enhances the severity of pancreatitis, making it an ideal model for studying the systemic inflammatory response in acute pancreatitis.
- Drug Evaluation: This model is used to test immune modulators, anti-inflammatory agents, antioxidants, and sepsis-related therapeutic agents. It is particularly valuable for evaluating drugs that target inflammatory cytokines, reduce systemic inflammation, and protect against multi-organ dysfunction during acute pancreatitis.
Measurements
We offer a variety of measurements for evaluating drug efficacy in rodent acute pancreatitis models, utilizing an array of advanced technologies, including but not limited to:
- General observations: Body weight, mortality rate, abdominal distension, signs of pain or discomfort.
- Cytokine profiling (e.g., ELISA): Expression levels of inflammatory mediators such as TNF-α, IL-6, IL-1β, and IL-10.
- Hematology analysis and serum biomarkers: Levels of serum amylase, lipase, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin.
- Gene/protein expression profiling via RT qPCR and Western blot techniques: Analysis of key genes or proteins involved in inflammation, apoptosis, and pancreatic injury, such as NF-κB, COX-2, and caspases.
- Histopathology: Assessment of pancreatic inflammation, necrosis, and edema in pancreatic tissue sections.
In addition to the established acute pancreatitis models, our expertise extends to the development of novel animal models tailored to specific research needs, based on literature and prior studies. Our scientific team is available to assist in experimental design, model selection, and data analysis, ensuring a customized and effective approach to your project at every stage.
Related Services
In addition to the acute pancreatitis model, we also offer a variety of other animal models for digestive system-related diseases, designed to evaluate the efficacy of different drugs and therapeutic strategies.
Advantages
- Comprehensive Expertise: We offer in-depth knowledge and experience in developing and evaluating various disease models, including gastric ulcers, colitis, and pancreatitis, ensuring high-quality preclinical research.
- Customized Solutions: Our team works closely with clients to design tailored experimental approaches, ensuring the best model selection for your specific research needs.
- Advanced Technologies: We use state-of-the-art technologies for accurate data analysis, from histological assessments to advanced molecular techniques like PCR, ELISA, and protein profiling.
- Reliable Results: With our rigorous quality control standards and scientifically validated models, we guarantee reliable and reproducible data, helping you advance your drug discovery process.
- End-to-End Support: From experimental design to data interpretation, we provide full project support, ensuring a smooth and efficient workflow for your research projects.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q1: What types of disease models do you offer for drug efficacy testing?
A1: We offer a wide range of rodent disease models for conditions like gastric ulcers, colitis, pancreatitis, and more, tailored to suit your research needs.
-
Q2: How do you ensure the quality of your preclinical research models?
A2: Our models undergo rigorous validation and quality control processes to ensure they replicate human disease conditions accurately and yield reliable results.
-
Q3: Can I customize a disease model for my specific research?
A3: Yes, we offer customized solutions based on your research requirements. Our team will work with you to select or develop the most suitable model.
-
Q4: What type of data can I expect from your models?
A4: We provide comprehensive data, including histological analysis, cytokine profiling, gene expression, and biochemical markers, to evaluate the therapeutic effects of your drug candidates.
-
Q5: Do you provide support throughout the research process?
A5: Yes, our team of experts provides full support, from experimental design and model selection to data interpretation and project analysis.
Published Data
This study investigated the effects of electroacupuncture (EA) at the Zusanli (ST-36) acupoint in experimental acute pancreatitis (AP) and the roles of the vagus nerve and α7nAChR on macrophages. Results showed that, compared to the NC group, plasma amylase levels were significantly elevated in the AP group after caerulein injection (Figures 2A, B). EA treatment notably reduced amylase levels at both 6 hours and 11 hours after caerulein injection. Histological analysis (Figures 2C–G) revealed severe pancreatic edema, leukocyte infiltration, and necrosis in AP mice. EA treatment alleviated these conditions, reducing edema, inflammation, and acinar necrosis. Vagotomy worsened pancreatitis severity, as indicated by increased plasma amylase levels and more severe histological damage in vagotomy mice. Cervical vagotomy also diminished the protective effects of EA. No significant difference was observed between vagotomy and vagotomy + EA groups in plasma amylase levels and histological injury. These findings suggest that EA’s protective effects are mediated through the vagus nerve.
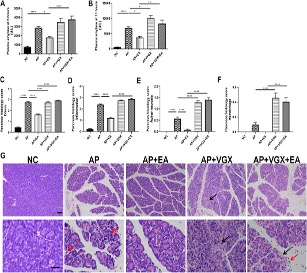 Fig. 2 EA alleviates the severity of caerulein AP mice.1
Fig. 2 EA alleviates the severity of caerulein AP mice.1
Reference
- Silva-Vaz, Pedro et al. "Murine Models of Acute Pancreatitis: A Critical Appraisal of Clinical Relevance." International Journal of Molecular Sciences vol. 20,11 2794. 7 Jun. 2019, doi:10.3390/ijms20112794. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
