ApoE*3 Transgenic (E3L) Mice Modeling & Pharmacodynamics Service
Creative Biolabs provides a comprehensive suite of well-established rodent models, including the ApoE*3 transgenic mice model, to accurately evaluate the efficacy of novel atherosclerosis interventions.
Introduction
Atherosclerosis, a chronic inflammatory disease of the arteries, is a leading cause of cardiovascular morbidity and mortality. It is characterized by the accumulation of lipids, immune cells, and fibrous tissue within arterial walls, leading to plaque formation and arterial stiffening. Understanding the complex interplay of genetic and environmental factors in its development is crucial for therapeutic innovation.
ApoE*3 Transgenic Mice Model
The ApoE*3 transgenic (E3L) mice model, also known as the APOE*3-Leiden mouse, is a valuable humanized animal model engineered to mimic human lipid metabolism and cardiovascular disease. By replacing the mouse APOE gene with human APOE3 under native regulatory elements, these mice exhibit a lipoprotein profile and dietary response closely resembling humans. This makes the E3L model an exceptional platform for studying hyperlipidemia, atherosclerosis development, and lipid-modulating therapies, while also serving as a critical baseline for understanding ApoE3's protective functions in neurological contexts.
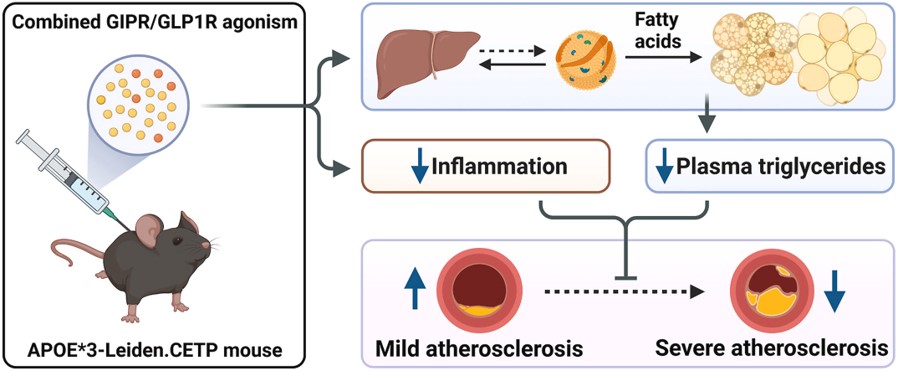 Fig.1 The study of the ApoE*3-Leiden mouse as a well-established mouse model for atherosclerosis development.1
Fig.1 The study of the ApoE*3-Leiden mouse as a well-established mouse model for atherosclerosis development.1
Model Construction Steps
The E3L mice model is generated using a targeted gene replacement strategy, involving homologous recombination to replace the murine APOE gene with the human APOE3 gene within the mouse genome. This ensures human ApoE3 protein expression under native mouse ApoE promoter and regulatory elements, leading to appropriate tissue-specific expression and physiological regulation.
01Targeting Vector Design
A specialized DNA construct (targeting vector) is engineered. This vector contains the human APOE3 gene flanked by sequences homologous to the mouse ApoE locus, along with selection markers.
02Embryonic Stem (ES) Cell Transfection
The targeting vector is introduced into mouse embryonic stem cells, where homologous recombination can occur, integrating the human APOE3 gene into the desired genomic location.
03Selection and Validation
ES cells that have successfully undergone homologous recombination are identified and expanded using selection markers. Genetic analysis (e.g., Southern blot, PCR) confirms the correct integration of the human APOE3 gene.
04Blastocyst Injection
Validated ES cells are then injected into early-stage mouse embryos (blastocysts).
05Chimeric Mouse Generation
The injected blastocysts are implanted into surrogate mothers, leading to the birth of chimeric mice, which contain cells derived from both the host embryo and the injected ES cells.
06Germline Transmission
Chimeric mice are bred with wild-type mice to identify offspring that have inherited the human APOE3 gene through their germline.
07Colony Expansion and Characterization
Mice carrying the human APOE3 gene are further bred to establish a stable colony. Comprehensive characterization confirms human ApoE3 expression and the desired lipoprotein phenotype.
Strengths and Limitations
Strengths:
- Physiological Relevance: Expresses human ApoE3, closely mimicking human lipoprotein metabolism and disease progression.
- Spontaneous Atherosclerosis: Develops atherosclerosis spontaneously on a Western-type diet, providing a robust model for intervention studies.
- Translational Value: Proven predictive value for clinical outcomes in drug and nutritional studies.
- Intact ApoE-LDLR Pathway: Maintains a functional lipoprotein remnant clearance pathway, allowing for evaluation of classic lipid-lowering interventions.
Limitations:
- Diet Dependence: Robust atherosclerosis development typically requires a high-fat, high-cholesterol (Western) diet.
- Not a Primary AD Model: While relevant for ApoE3 function, it does not spontaneously develop Alzheimer's-like neurodegeneration unless crossed with specific AD-related mutations.
- Species-Specific Differences: Despite humanization, some inherent mouse physiological differences may persist.
Evaluation Platform
Creative Biolabs offers a comprehensive evaluation platform to thoroughly assess the efficacy of compounds and interventions in the E3L mice model. Our state-of-the-art facilities and expert team enable detailed biochemical, molecular, cellular, and histopathological analyses. We employ advanced imaging techniques and behavioral tests (where applicable) to provide a holistic understanding of disease progression and therapeutic impact.
Key Test Parameters:
- Biochemical: Plasma lipid profiles (total cholesterol, HDL-C, LDL-C, triglycerides), inflammatory markers (e.g., cytokines, chemokines), glucose, insulin.
- Molecular: Gene expression (RT-qPCR) of key lipid metabolism genes, inflammatory mediators, and neuronal markers; protein expression (Western blot, ELISA).
- Cellular: Macrophage foam cell formation, immune cell phenotyping (flow cytometry), endothelial cell activation.
- Histopathological: Atherosclerotic plaque burden (aortic root, whole aorta), plaque composition (lipid content, collagen, smooth muscle cells, macrophages), liver steatosis, arterial remodeling.
- Imaging: Ultrasound or MRI for non-invasive plaque quantification and vascular function.
- Behavioral: Cognitive assessments (e.g., Morris Water Maze, Y-maze) if neurological endpoints are relevant.
Applications
- Disease Modeling: Simulating and studying hyperlipidemia, atherosclerosis progression, insulin resistance, and metabolic syndrome. It also serves as a crucial control for neurodegeneration studies involving ApoE4-driven pathologies.
- Therapeutic Evaluation: Extensively used for evaluating a broad spectrum of interventions, including lipid-lowering, anti-inflammatory, anti-atherosclerotic, anti-diabetic, and neuroprotective drugs, encompassing small molecules, biologics, nutritional interventions, and gene therapies.
- Mechanism Elucidation: Providing critical insights into disease mechanisms, allowing researchers to assess the efficacy and safety of novel compounds and treatments across cardiovascular, metabolic, and neurological research domains.
Related Atherosclerosis Models
- ApoE-/- Mice Model
- Low-Density Lipoprotein Receptor-Deficient Mice (LDLR-/-) Model
- Fatty Zucker Rats Model
- Carotid Artery Endothelial Denudation Model
- High-Fat-Diet (HFD) & CHOL-Induced Aorta Atherosclerosis Model
- Blood Flow-Induced Arterial Intimal Thickening Model
Our Advantages
- Unrivaled Physiological Relevance: Our models precisely mimic human ApoE3 biology, ensuring highly translatable research outcomes.
- Robust Baseline: Provides the gold standard "normal" baseline for accurate comparative studies against disease models.
- Proven Translational Value: Backed by over 150 industry studies demonstrating predictive clinical relevance.
- Accelerated Drug Discovery: Facilitates efficient identification and validation of therapeutic targets for multiple diseases.
- Reproducibility & Reliability: Rigorous quality control ensures consistent, dependable experimental results.
- Expert Scientific Support: Our seasoned biologists offer comprehensive guidance throughout your research journey.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
Contact Us
Creative Biolabs is committed to advancing your research with high-quality, physiologically relevant animal models and expert scientific services. We provide comprehensive solutions for evaluating novel therapies across cardiovascular, metabolic, and neurological domains. Contact us today to discuss how the E3L mice model can accelerate your next breakthrough.
FAQs
-
Q1: What distinguishes the E3L mouse from other hyperlipidemia models?
A: The E3L model is unique because it expresses human ApoE3, rather than endogenous mouse ApoE or a complete ApoE knockout. This humanization leads to a lipoprotein metabolism that closely mirrors human physiology, including the formation of VLDL remnants and an LDL-like lipoprotein profile, making it highly relevant for studying human-specific lipid disorders and atherosclerosis.
-
Q2: How does the ApoE*3-Leiden.CETP variant enhance the model's utility?
A: The APOE*3-Leiden.CETP mouse is a double transgenic model that additionally expresses human Cholesteryl Ester Transfer Protein (CETP). CETP plays a significant role in human cholesterol metabolism by facilitating the transfer of cholesteryl esters from HDL to ApoB-containing lipoproteins. This variant provides an even more human-like lipoprotein profile, particularly regarding HDL metabolism, making it ideal for evaluating therapies that target CETP or aim to modulate HDL levels.
-
Q3: Is it possible to assess cognitive function in the E3L mice?
A: Yes, cognitive function can be assessed in E3L mice, especially when investigating the impact of metabolic or inflammatory interventions on brain health, or when using them as controls for neurodegenerative models. Standard behavioral tests such as the Morris Water Maze, Y-maze, or novel object recognition can be employed to evaluate learning, memory, and spatial cognition.
-
Q4: How do you ensure the genetic stability and quality of the E3L mouse colony?
A: Creative Biolabs maintains rigorous quality control measures for our E3L mouse colonies. This includes routine genetic monitoring through genotyping to confirm the presence and stability of the human ApoE3 transgene, health monitoring to ensure a pathogen-free environment, and careful breeding strategies to maintain genetic purity and phenotypic consistency across generations.
-
Q5: Can you assist with custom study design for the E3L model?
A: Absolutely. Our team of experienced scientists specializes in preclinical study design and can provide comprehensive consultation. We work closely with clients to tailor experimental protocols, including diet, duration, dosing regimens, and endpoint selection, to ensure the study aligns perfectly with their specific research goals and maximizes data relevance.
-
Q6: What is the significance of the "intact ApoE–LDLR pathway" in the E3L model?
A: The intact ApoE–LDLR pathway refers to the functional interaction between ApoE and the LDLR family, which is crucial for clearing triglyceride-rich lipoprotein remnants from circulation. Unlike some other atherosclerosis models that lack functional LDLR, the E3L model retains this pathway, making it highly responsive to classic lipid-lowering interventions that modulate LDLR activity, thus increasing its translational relevance.
Published Data
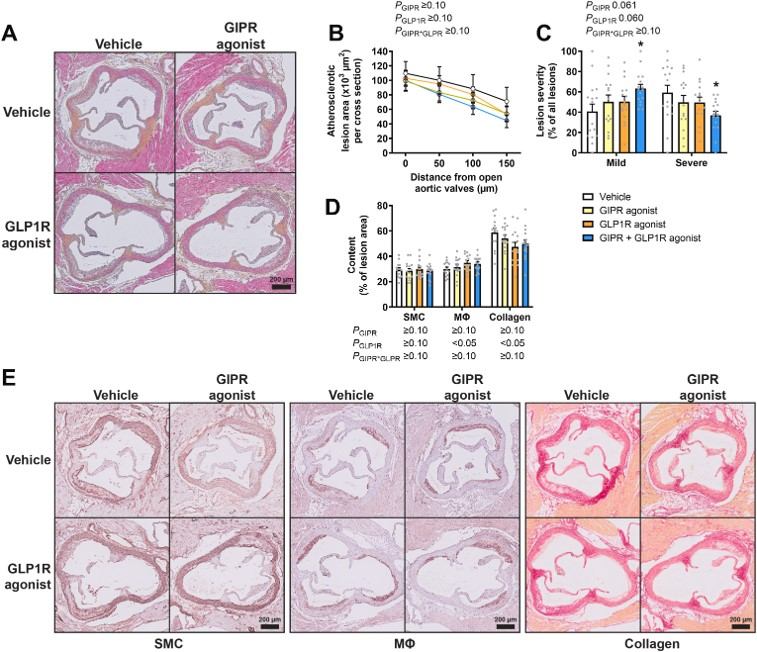 Fig.2 Combined GIPR/GLP1R agonism attenuates atherosclerotic lesion severity.1
Fig.2 Combined GIPR/GLP1R agonism attenuates atherosclerotic lesion severity.1
The translational utility of the ApoE*3-Leiden mouse model is well-documented in scientific literature. This article investigated the effects of combined GIPR/GLP1R agonism on atherosclerosis in APOE*3-Leiden.CETP mice. The project results demonstrated that combined agonism significantly attenuated the development of severe atherosclerotic lesions, reduced systemic low-grade inflammation, and markedly lowered plasma triglyceride levels by increasing VLDL turnover. This research highlights the model's predictive value for evaluating novel cardiometabolic therapies.
Reference
- van Eenige, Robin et al. "Combined glucose-dependent insulinotropic polypeptide receptor and glucagon-like peptide-1 receptor agonism attenuates atherosclerosis severity in APOE*3-Leiden.CETP mice." Atherosclerosis vol. 372 (2023): 19-31. Distributed under Open Access license CC BY 4.0, without modification. DOI: 10.1016/j.atherosclerosis.2023.03.016
For Research Use Only.
