Sodium Laurate induced Vasculitis Modeling & Pharmacodynamics Service
Introduction
Peripheral vascular disease (PVD) encompasses a range of conditions affecting blood vessels outside of the heart and brain, often leading to reduced blood flow to the limbs. Inflammatory processes frequently play a critical role in the initiation and progression of these debilitating diseases, contributing to pain, tissue damage, and impaired function. Understanding these complex mechanisms is crucial for developing effective therapies.
Creative Biolabs is dedicated to advancing PVD research by providing a variety of well-established and meticulously validated in vivo models, enabling comprehensive evaluation of novel therapeutic candidates.
Sodium Laurate-Induced Vasculitis Model
The sodium laurate-induced vasculitis model is a widely recognized and highly reproducible preclinical model used to study acute vascular inflammation and its associated pathologies. It involves the localized administration of sodium laurate, a fatty acid salt, to induce a rapid and robust inflammatory response within specific peripheral blood vessels, typically the femoral artery. This model effectively mimics key features of human vasculitis, including endothelial injury, leukocyte infiltration, and pro-inflammatory cytokine release.
Model Construction Steps
The construction of the sodium laurate-induced vasculitis model primarily involves a localized chemical injury to a target blood vessel in rodents, most commonly rats or mice. This approach ensures a controlled and consistent inflammatory response, allowing for precise experimental manipulation and robust data generation.
01Animal Preparation
Healthy rodents, typically Wistar rats or C57BL/6 mice, are selected at an appropriate age and weight. Animals are acclimatized to the facility environment and fasted prior to the procedure.
02Anesthesia
Animals are anesthetized using a suitable anesthetic agent (e.g., isoflurane or ketamine/xylazine) to ensure immobility and minimize discomfort throughout the surgical procedure.
03Surgical Exposure
A small incision is made to expose the target blood vessel, most commonly the femoral artery in the hind limb. Meticulous surgical technique is employed to minimize tissue damage and ensure precise access to the vessel.
04Sodium Laurate Administration
A precise volume and concentration of sodium laurate solution (e.g., 5% sodium laurate, 50 μL) is carefully injected peri-vascularly or directly into the vessel lumen. The injection site is then briefly compressed to ensure localized exposure and prevent leakage.
05Wound Closure and Recovery
The surgical incision is closed using sterile sutures or clips, and the animals are allowed to recover under close monitoring. Analgesia is administered as needed to manage post-operative pain.
06Observation Period
Animals are monitored daily for the development of visible signs of vasculitis, such as paw swelling, color changes, or gangrene. The typical observation period extends for 7 to 14 days, depending on the study objectives.
Strengths and Limitations
Strengths:
- High Reproducibility: Consistent and predictable lesion development due to controlled induction.
- Rapid Induction: Fast onset of inflammatory symptoms, enabling efficient compound screening.
- Cost-Effectiveness: Relatively straightforward and less resource-intensive compared to complex genetic models.
Limitations:
- Acute Nature: Primarily models acute inflammatory phases, less suited for chronic or autoimmune vasculitis without modifications.
- Localized Response: Induces localized inflammation, not a systemic autoimmune vasculitis.
Evaluation Platform
Creative Biolabs offers comprehensive evaluation platforms to assess therapeutic agent efficacy in the Sodium Laurate-Induced Vasculitis Model. Our capabilities, spanning biochemical, molecular, cellular, histopathological, and imaging analyses, provide a holistic view of disease progression and treatment response.
Key Test Parameters:
- Gross Pathological Scoring: Visual assessment of limb ischemia, swelling, and gangrene severity.
- Histopathology: Hematoxylin and Eosin (H&E) staining for inflammatory cell infiltration, vessel wall thickening, and thrombosis. Special stains for collagen (fibrosis) or fibrin (thrombi).
- Biochemical Markers (Plasma/Serum): Quantitation of pro-inflammatory cytokines (e.g., Urocortin, PGE2, TNF-α, IL-6), soluble adhesion molecules (sICAM-1, sVCAM-1), and coagulation parameters (e.g., PT, TT, Fibrinogen, platelet counts).
- Molecular Analysis (Tissue): Gene and protein expression analysis (Western Blot, qPCR, ELISA) of key inflammatory mediators (e.g., COX-2, ICAM-1), adhesion molecules, and signaling pathway components.
- Immunohistochemistry/Immunofluorescence: Localization and quantification of specific cell types (e.g., macrophages, neutrophils, T cells) and protein expression within the vascular tissue.
Applications
- Diseases Simulation: Primarily used to simulate aspects of acute vasculitis, including thromboangiitis obliterans (TAO), and to study the inflammatory components of peripheral artery disease.
- Drug Classes Evaluation: Ideal for assessing the efficacy of novel anti-inflammatory agents, immunosuppressants, anti-thrombotic compounds, and endothelial protective therapies.
- Treatments Investigation: Applicable for evaluating small molecule inhibitors, therapeutic antibodies, gene therapies, and traditional medicine formulations.
- Mechanistic Studies: Crucial for elucidating the roles of specific cytokines, chemokines, adhesion molecules, and cellular pathways in vascular inflammation.
- Gender Disparity Research: Particularly valuable for investigating sex-specific differences in disease susceptibility and progression, as well as the influence of sex hormones.
Related Peripheral Vascular Disease Models
- Femoral Artery Ligation induced Lower Limb Peripheral Vascular Disease Model
- Sodium Laurate induced Lower Limb Gangrene Model
Our Advantages
- Optimized Protocols: Meticulously validated protocols ensure highly reproducible and translatable results.
- Expert Team: Skilled biologists, pharmacologists, and pathologists dedicated to scientific excellence.
- Advanced Facilities: State-of-the-art labs with cutting-edge analytical techniques.
- Customized Solutions: Flexible study designs tailored to your unique research objectives.
- Actionable Insights: Comprehensive data packages provide clear, actionable insights for drug development.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
Contact Us
Creative Biolabs stands ready to empower your PVD research. By providing robust in vivo models and comprehensive evaluation services, we help de-risk your therapeutic candidates. We encourage you to contact us today for a confidential consultation to explore how our expertise can accelerate your drug discovery efforts.
FAQs
-
Q1: Can this model be used to study chronic vasculitis or only acute inflammation?
A: This model primarily induces acute inflammation, typically resolving within two weeks. It excels for acute interventions but is not designed for chronic or autoimmune vasculitis. For such studies, modifications or alternative models may be necessary, which our experts can discuss.
-
Q2: Is the vasculitis induced by sodium laurate localized or systemic?
A: Sodium laurate-induced vasculitis is mainly localized to the injection site, usually a peripheral artery. While systemic inflammation markers may elevate, primary changes are confined to the treated limb. This localized nature is ideal for studying direct vascular injury without systemic complexities.
-
Q3: How does gender influence the outcomes in the sodium laurate-induced vasculitis model?
A: This model shows significant gender disparity; males often exhibit more severe symptoms. This difference is linked to sex hormones, particularly androgens, which can exacerbate inflammation and urocortin production. This makes the model valuable for gender-specific research.
-
Q4: Can this model be used to evaluate combination therapies?
A: Yes, this model is well-suited for combination therapy evaluation. Its robust, reproducible inflammatory response provides a clear baseline to measure synergistic or additive effects. This helps optimize dosing and identify effective drug combinations.
-
Q5: Are there options for customizing the model, such as varying the severity of vasculitis?
A: Yes, the model offers significant customization. We can adjust sodium laurate concentration, volume, and injection technique to modulate vasculitis severity. This enables dose-response studies or targeting different inflammatory stages.
Published Data
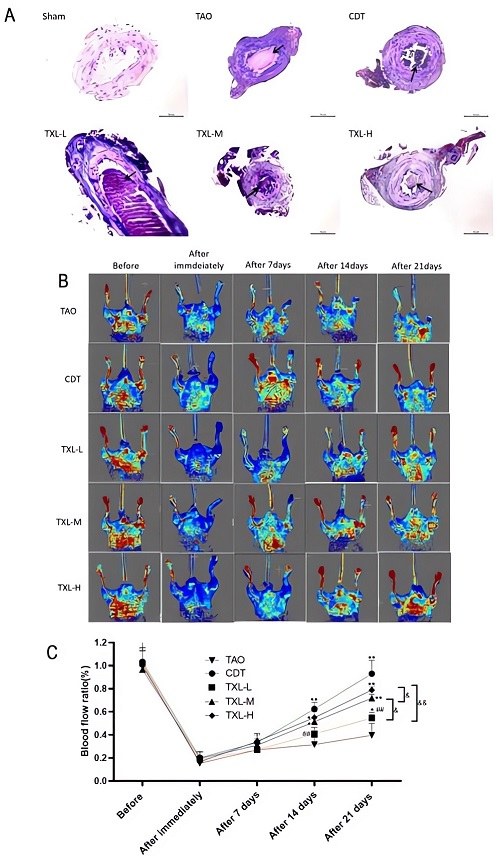 Fig.1 Visualization of femoral artery thrombosis in TAO mice and the effect of TXL on blood flow recovery in the lower limbs of TAO mice.1
Fig.1 Visualization of femoral artery thrombosis in TAO mice and the effect of TXL on blood flow recovery in the lower limbs of TAO mice.1
In this study, researchers utilized a mouse model of sodium laurate-induced TAO to evaluate the efficacy of Tongxinluo (TXL), a traditional Chinese medicine. The project successfully demonstrated that TXL exhibited protective effects, modulating inflammation, oxidative stress, and coagulation pathways in the mouse model of thromboangiitis obliterans. This case exemplifies how our robust models facilitate the discovery and mechanistic understanding of new treatments for complex PVD pathologies.
Reference
- Gu, Jiaojiao, and Huailin Gao. 'Protective effects and potential mechanism of To ngxinluo on mouse with thromboangiitis obliterans induced by sodium laurate." ScienceOpen Preprints (2022). Distributed under Open Access license CC BY 4.0, without modification. DOI: 10.14293/S2199-1006.1.SOR-.PPDZMLW.v1.
For Research Use Only.
