Apolipoprotein E Deficient (ApoE-/-) Mice Modeling & Pharmacodynamics Service
Creative Biolabs provides a variety of well-established preclinical models, including the ApoE-/- mouse, to accurately evaluate the efficacy of novel anti-atherosclerotic compounds and therapeutic strategies.
Introduction
Atherosclerosis, a chronic inflammatory disease of the arteries, is the primary underlying cause of cardiovascular diseases such as heart attack and stroke. It involves the accumulation of lipids, inflammatory cells, and fibrous tissue within arterial walls, leading to plaque formation and arterial narrowing. Understanding its complex progression is vital for developing effective therapies.
Apolipoprotein E Deficient Mice Model
The apolipoprotein E deficient mouse (ApoE-/-) model represents a significant breakthrough in atherosclerosis research by providing a spontaneously developing, human-like atherosclerotic phenotype, enabling the study of human-like plaque development in a controlled in vivo setting.
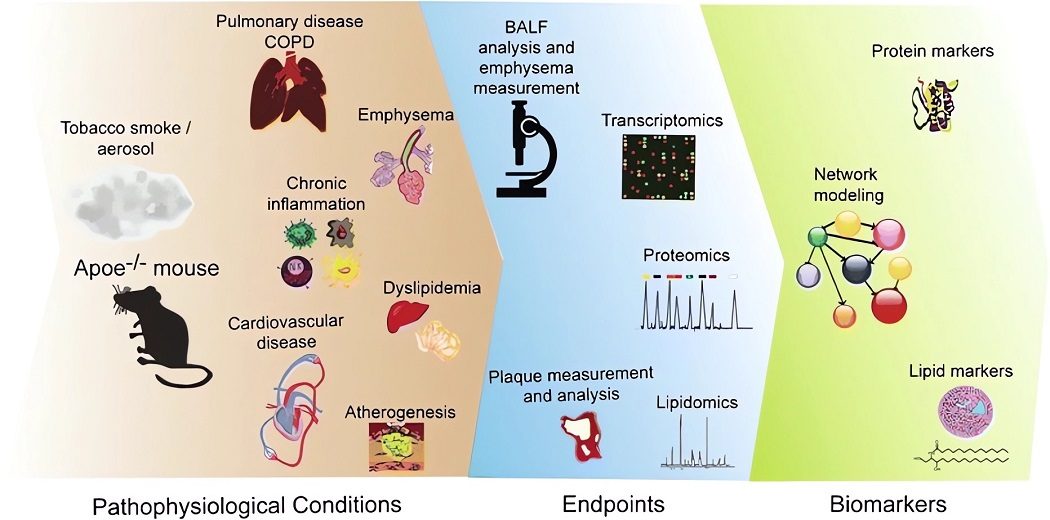 Fig.1 The pathophysiological events and endpoints in ApoE-/-mouse model.1,3
Fig.1 The pathophysiological events and endpoints in ApoE-/-mouse model.1,3
Model Construction Steps
The ApoE-/- mouse model is meticulously generated through targeted gene disruption via homologous recombination in embryonic stem (ES) cells. The construction process typically involves several precise steps:
01ES Cell Isolation
Pluripotent ES cells are first isolated from mouse blastocysts.
02Gene Targeting Construct Design
A specialized DNA construct is engineered. This construct contains sequences homologous to the ApoE gene, designed to facilitate precise recombination, along with a selectable marker gene (e.g., for antibiotic resistance) to identify successfully modified cells.
03Electroporation and Selection
The gene targeting construct is introduced into the ES cells via electroporation. Cells that have successfully undergone homologous recombination and incorporated the construct are then selected using the marker gene.
04Blastocyst Injection
The genetically modified ES cells are injected into early-stage mouse embryos (blastocysts).
05Chimeric Mouse Generation
These injected blastocysts are then implanted into pseudopregnant female mice, leading to the birth of chimeric offspring, which carry both modified and unmodified cells.
06Germline Transmission and Breeding
Chimeric mice are bred to wild-type mice to identify individuals that have transmitted the modified ApoE gene through their germline.
07Homozygous Line Establishment
Heterozygous offspring (carrying one copy of the disrupted ApoE gene) are then intercrossed to produce homozygous ApoE-/- mice, which completely lack functional ApoE.
Strengths and Limitations
Strengths:
- Spontaneous Disease Development: ApoE-/- mice spontaneously develop severe hyperlipidemia and progressive, human-like atherosclerotic lesions, even on a standard diet.
- Human-like Plaque Morphology: Plaques progress through stages similar to human disease, from fatty streaks to advanced fibrous plaques, including vulnerable characteristics in older mice.
- Dietary Modulability: Dietary interventions, like high-fat diets, significantly accelerate atherosclerosis progression, offering flexible study designs.
- Versatile Research Platform: It's a gold standard for studying atherogenesis, evaluating lipid-lowering and anti-inflammatory therapies, and exploring dyslipidemia's interplay with metabolic conditions.
Limitations:
- Coronary Atherosclerosis Complexity: While excellent for aortic atherosclerosis, inducing severe coronary atherosclerosis often requires additional genetic modifications (e.g., crossing with LDLR deficient mice), adding complexity to breeding strategies.
Evaluation Platform
Creative Biolabs offers a comprehensive evaluation platform to thoroughly assess therapeutic efficacy in ApoE-/- mice, encompassing:
- Biochemical Analyses: Detailed lipid profiling and lipoprotein analysis.
- Molecular Assays: Assessment of gene expression and protein analysis.
- Cellular Studies: Investigation of foam cell formation and smooth muscle cell proliferation.
- Histopathological Assessments: Advanced evaluation of plaque size, composition, stability, vulnerable plaque characteristics, and novel histomorphometric analysis of coronary lesions.
- Imaging and Functional Studies: In vivo imaging, behavioral assays for cognitive function, and evaluation of cardiac and carotid artery function.
Applications
- Disease Simulation: Used to simulate and study the progression of atherosclerosis, hypercholesterolemia, dyslipidemia, and related cardiovascular pathologies. It is also relevant for understanding aspects of neurodegenerative diseases (e.g., Alzheimer's disease), diabetes complications, Non-alcoholic Fatty Liver Disease (NAFLD)/Non-alcoholic Steatohepatitis (NASH), and kidney disease.
- Drug Evaluation: Ideal for evaluating the efficacy of novel lipid-lowering agents (e.g., PCSK9 inhibitors, statins), anti-inflammatory drugs, and gene therapies targeting lipid metabolism or vascular inflammation.
- Treatment Modalities: Enables the assessment of various therapeutic interventions, including dietary modifications, lifestyle interventions, and surgical or device-based approaches.
- Mechanistic Studies: Provides a robust platform for unraveling the cellular and molecular mechanisms underlying disease initiation, progression, and regression, including studies on endothelial dysfunction, vascular remodeling, and plaque stability.
Related Atherosclerosis Models
- Low-Density Lipoprotein Receptor-Deficient Mice (LDLR-/-) Model
- ApoE*3 Transgenic (E3L) Mice Model
- Fatty Zucker Rats Model
- Carotid Artery Endothelial Denudation Model
- High-Fat-Diet (HFD) & CHOL-Induced Aorta Atherosclerosis Model
- Blood Flow-Induced Arterial Intimal Thickening Model
Our Advantages
- Unrivaled Expertise: Our team of seasoned biologists and pharmacologists provides expert consultation, guiding you from experimental design to data interpretation.
- High-Quality Models: We maintain meticulously characterized ApoE-/- colonies, ensuring consistent genetic background and reproducible phenotypic expression.
- Comprehensive Phenotyping: Access a full suite of advanced phenotyping capabilities, delivering in-depth insights into disease mechanisms and therapeutic effects.
- Customized Solutions: We collaborate closely to design and execute studies tailored precisely to your unique research objectives and timelines.
- Translational Impact: Our studies are designed to generate data that is highly predictive of human outcomes, accelerating your journey from discovery to clinical application.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
Contact Us
Creative Biolabs is dedicated to accelerating your research through high-quality preclinical services. We provide comprehensive support, from model selection and study design to data analysis and reporting. Contact us today to discuss how our expertise with the ApoE-/- model can empower your next groundbreaking study.
FAQs
-
Q1: How do you ensure the reproducibility and reliability of studies conducted with ApoE-/- mice?
A: We prioritize data quality and reproducibility. We achieve this by maintaining meticulously characterized ApoE-/- colonies with consistent genetic backgrounds. Our stringent protocols for animal husbandry, experimental procedures, and comprehensive phenotyping minimize variability, ensuring robust and reliable results for your research.
-
Q2: What is the typical timeline for observing advanced atherosclerotic lesions in ApoE-/- mice?
A: Advanced atherosclerotic lesions can be observed relatively early in ApoE-/- mice. Significant plaque development is evident as early as 2-3 months of age, even when the mice are maintained on a regular chow diet. This makes it a time-efficient model for studying disease progression and therapeutic interventions.
-
Q3: Can you assist with custom experimental designs for complex research questions involving ApoE-/- mice?
A: Absolutely. Our team of experienced scientists specializes in custom study design and execution. We work collaboratively with our clients to develop tailored experimental protocols that address specific, complex mechanistic investigations or multi-faceted research objectives, ensuring optimal outcomes.
-
Q4: What specific phenotyping capabilities do you offer for atherosclerosis studies in ApoE-/- mice?
A: Our comprehensive phenotyping capabilities include detailed lipid and lipoprotein analysis, advanced histological assessment of plaque size, composition, and stability, characterization of vulnerable plaque features, and novel histomorphometric analysis of coronary arteries. We also offer in vivo imaging and functional assessments of cardiac and carotid artery health.
-
Q5: Does Creative Biolabs provide regulatory-compliant services for preclinical studies using ApoE-/- mice?
A: Yes, Creative Biolabs understands the importance of regulatory compliance in preclinical drug development. We offer GLP-compliant services for studies utilizing the ApoE-/- model, ensuring that your research data meets the necessary quality and integrity standards for regulatory submissions and accelerates your path to clinical trials.
Published Data
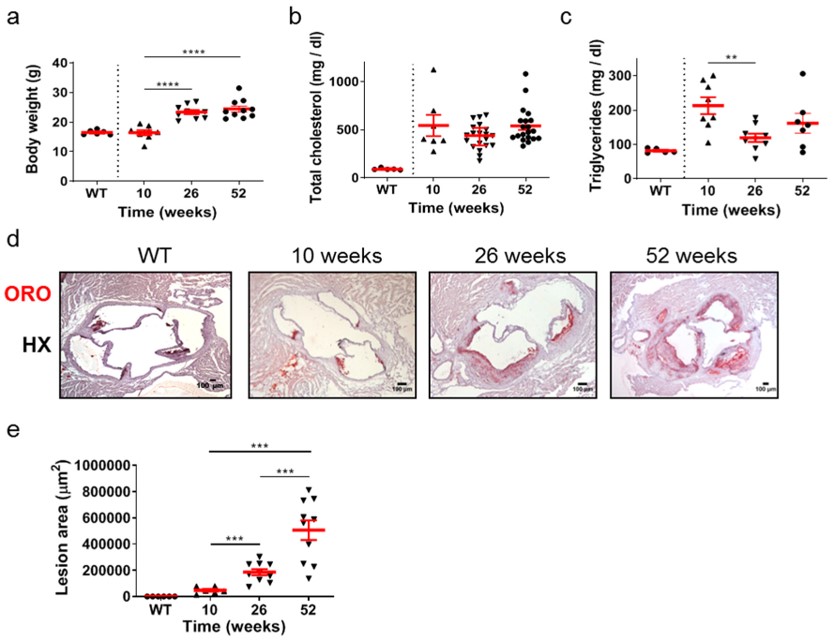 Fig.2 The highly dynamic process of atherosclerosis in ApoE-/- mice.2,3
Fig.2 The highly dynamic process of atherosclerosis in ApoE-/- mice.2,3
In a compelling demonstration of the ApoE-/- model's utility, a study provided a detailed characterization of atherosclerosis progression. Researchers showed that in ApoE-/- mice on a normal chow diet, aortic valve lesions evolve from cellular, macrophage-rich plaques at 26 weeks to acellular, lipid-rich, and more necrotic phenotypes by 52 weeks. This progression was marked by enhanced lipid deposition, calcification, and increased plaque vulnerability, highlighting the model's capacity to reveal critical aspects of advanced atherosclerotic disease.
References
- Lo Sasso, Giuseppe et al. "The Apoe(-/-) mouse model: a suitable model to study cardiovascular and respiratory diseases in the context of cigarette smoke exposure and harm reduction." Journal of translational medicine vol. 14,1 146. 20 May. 2016. https://doi.org/10.1186/s12967-016-0901-1
- Kotsovilis, Sotirios et al. "Comprehensive Analysis of 1-Year-Old Female Apolipoprotein E-Deficient Mice Reveals Advanced Atherosclerosis with Vulnerable Plaque Characteristics." International journal of molecular sciences vol. 25,2 1355. 22 Jan. 2024. https://doi.org/10.3390/ijms25021355
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
