Ascending Aortic Arch Constriction induced Post-Pressure Overload Heart Failure Modeling & Pharmacodynamics Service
At Creative Biolabs, we specialize in providing a diverse array of meticulously established preclinical HF models, specifically designed to rigorously assess the effectiveness of emerging therapies and accelerate their development.
Introduction
Heart failure (HF) represents a critical public health concern, characterized by the heart's compromised ability to circulate blood efficiently. This pervasive condition leads to significant morbidity and mortality globally. Advancements in treatment are continuously sought, yet the pressing demand for innovative therapeutic solutions remains.
Ascending Aortic Arch Constriction-Induced Post-Pressure Overload HF Model
The ascending aortic arch constriction (AoS) model is meticulously engineered to recapitulate the pathological progression of pressure overload-induced HF observed in human patients. This surgical model induces a chronic increase in afterload on the left ventricle, compelling the heart to labor against elevated resistance.
 Fig.1 Surgical techniques for the AoS rat model of HF.1
Fig.1 Surgical techniques for the AoS rat model of HF.1
Model Construction Steps
The model's construction involves a precise surgical intervention to create a defined constriction in the ascending aorta. This procedure initiates a cascade of events leading to HF.
01Anesthesia and Thoracotomy
Animals are first anesthetized to ensure their comfort and immobility. A meticulous thoracotomy is then performed to gain access to the thoracic cavity.
02Aortic Isolation
The ascending aorta is carefully isolated from surrounding tissues, ensuring minimal disturbance to adjacent structures.
03Constriction Application
A non-absorbable suture or micro-clip is precisely positioned around the ascending aorta. The constriction is tightened to a predetermined diameter, typically against a standardized wire gauge to ensure consistent pressure overload across subjects. This precise stenosis creates a fixed, elevated pressure gradient.
04Closure and Recovery
Following successful constriction, the chest cavity is carefully closed, and the animal is allowed to recover under close postoperative monitoring.
Strengths and Limitations
Strengths:
- Physiological Relevance: The AoS model accurately mimics the chronic pressure overload seen in clinical conditions like hypertension or aortic stenosis, leading to a progressive and well-characterized disease course from compensatory hypertrophy to decompensated HF.
- Reproducibility: When performed by skilled surgical teams, the model exhibits high reproducibility in inducing consistent cardiac hypertrophy and dysfunction, enabling robust comparisons between treatment groups.
- Comprehensive Pathophysiology: It faithfully replicates key pathological features of human HF, including myocardial hypertrophy, fibrosis, ventricular dilation, contractile dysfunction, and neurohumoral activation.
Limitations:
- Surgical Complexity: The procedure requires significant surgical expertise to ensure consistency and minimize variability, impacting animal welfare and experimental outcomes if not executed precisely.
- Irreversibility: A primary limitation of the standard AoS model is the difficulty in reversing the pressure overload once induced without an additional surgical intervention. This can present challenges for studies specifically designed to investigate the active reversal of established HF, though emerging techniques in related models aim to address this.
Evaluation Platform
Creative Biolabs provides a robust and multi-faceted evaluation platform to thoroughly characterize the cardiac phenotype induced by the AoS model and assess therapeutic efficacy. Our state-of-the-art capabilities encompass:
- Imaging:
Echocardiography: Non-invasive, serial assessment of left ventricular function (ejection fraction, fractional shortening), dimensions (LV end-diastolic/systolic diameter), and wall thickness.
Hemodynamics (Pressure-Volume Loop Analysis): Invasive, high-fidelity measurements of cardiac contractility (dP/dtmax), relaxation (dP/dtmin), end-systolic elastance (Ees), and preload recruitable stroke work (PRSW).
- Histopathology:
Detailed analysis of myocardial hypertrophy (cardiomyocyte cross-sectional area), fibrosis (collagen volume fraction via Masson's trichrome or picrosirius red staining), and inflammatory cell infiltration.
- Biochemical and Molecular Analysis:
Quantification of circulating biomarkers (e.g., BNP, troponin), gene expression (qPCR, RNA-Seq), and protein levels (Western blot, ELISA) related to cardiac stress, remodeling, and inflammation.
- Cellular Analysis:
Assessment of cardiomyocyte apoptosis or proliferation, and fibroblast activation.
Applications
Simulated Diseases: Primarily, it models pressure overload-induced cardiac hypertrophy and its progression to dilated cardiomyopathy and HF with preserved or reduced ejection fraction (HFpEF/HFrEF). It also provides insights into the mechanisms underlying pathological cardiac remodeling and myocardial fibrosis.
Drug Evaluation: The model is highly effective for assessing the efficacy of novel compounds targeting various aspects of HF pathophysiology, including anti-hypertrophic agents, anti-fibrotic drugs, modulators of myocardial contractility, and agents influencing neurohumoral activation.
Treatment Modalities: Beyond pharmacological agents, the AoS model can be adapted to evaluate gene therapies, cell-based therapies, and device-based interventions aimed at improving cardiac function and preventing disease progression.
Related Heart Failure Models
PA Constriction induced Right HF Model
Abdominal Aortic Stenosis induced Left HF Model
DOCA & Salt induced Left HF Model
Adriamycin induced Left HF Model
Our Advantages
- Years of Expertise: Deep, specialized knowledge in cardiovascular research and robust model execution.
- Rigorous Surgical Consistency: Our highly trained surgical teams ensure precise, reproducible AoS induction, minimizing experimental variability.
- Comprehensive Characterization: State-of-the-art imaging, hemodynamic, histopathological, and molecular analyses provide a holistic view of cardiac health.
- Tailored Study Designs: We collaborate closely to create customized protocols, aligning with your unique research objectives and accelerating your discovery.
- Uncompromising Quality: Strict adherence to animal welfare and regulatory guidelines ensures reliable, high-quality, and reproducible data.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
Contact Us
Creative Biolabs invites you to contact our scientific team. Discuss your specific research needs and explore how our unparalleled expertise in the AoS-induced post-pressure overload HF model can significantly advance your cardiovascular drug development efforts.
FAQs
-
Q1: What defines the "gold standard" status of the AoS model in HF research?
A: The AoS model is widely recognized as a gold standard due to its remarkable ability to replicate the progressive pathological changes observed in human pressure overload-induced HF, including adaptive hypertrophy, maladaptive remodeling, fibrosis, and eventual cardiac dysfunction. Its predictable and well-characterized disease progression makes it an excellent platform for mechanistic studies and therapeutic evaluations.
-
Q2: How do you ensure the consistency and reproducibility of the AoS surgical procedure?
A: Our surgical teams possess extensive experience and undergo rigorous training specific to the AoS procedure. We employ standardized protocols, including precise measurement of the constriction diameter using calibrated gauges, and utilize advanced microsurgical techniques, all contributing to minimized variability and enhanced reproducibility across studies.
-
Q3: Can the AoS model differentiate between HFpEF and HFrEF?
A: While primarily known for inducing HFrEF, the AoS model can indeed exhibit characteristics relevant to both HFpEF and HFrEF, depending on the severity and duration of the pressure overload. Early phases may present features akin to HFpEF (e.g., diastolic dysfunction, concentric hypertrophy), gradually progressing to HFrEF (systolic dysfunction, chamber dilation) over time, allowing researchers to investigate both phenotypes.
-
Q4: What types of therapeutic interventions can be effectively evaluated using the AoS model?
A: The AoS model is highly versatile for evaluating a broad spectrum of interventions, including novel small molecules, biologics, gene therapies, and even device-based approaches. It is particularly well-suited for agents designed to prevent or reverse myocardial hypertrophy, mitigate fibrosis, improve cardiac contractility, or modulate neurohumoral pathways implicated in HF.
-
Q5: Is it possible to investigate the reversibility of HF in studies utilizing the AoS model?
A: The conventional AoS model primarily focuses on inducing and studying the progression of HF under continuous pressure overload. While direct, non-invasive reversal of the constriction is not a standard feature of the AoS model itself, Creative Biolabs can discuss alternative study designs or emerging methodologies in related models, such as staged interventions, to explore aspects of cardiac recovery.
-
Q6: Can you customize the AoS study design to meet specific client research objectives?
A: Absolutely. Customization is a cornerstone of our service. We engage in thorough consultations with our clients to understand their unique research questions, enabling us to tailor every aspect of the AoS study protocol—from animal strain and surgical severity to assessment time points and readouts—ensuring maximal relevance to your therapeutic goals.
Published Data
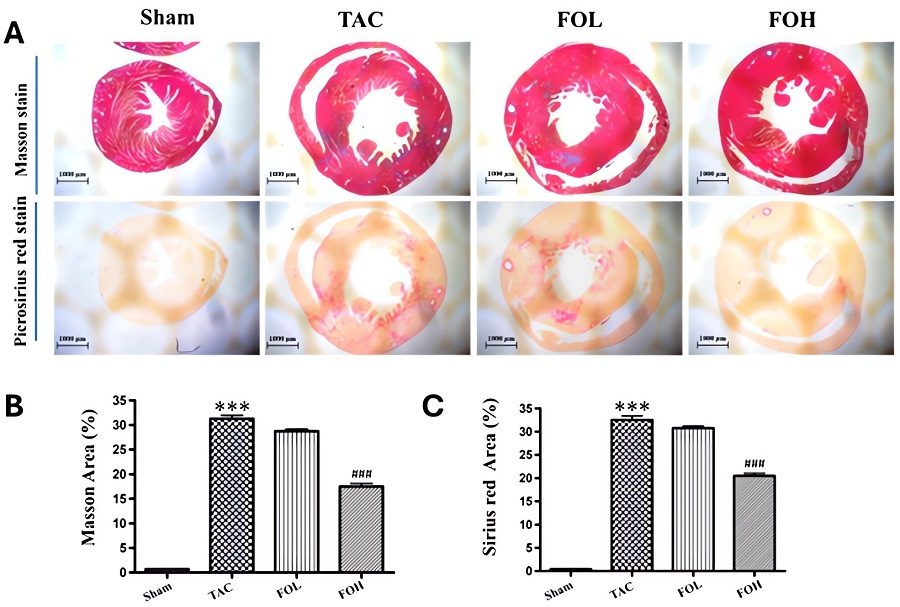 Fig.2 Effects of fucoidan on histopathological changes in a TAC induced HF mouse model.2
Fig.2 Effects of fucoidan on histopathological changes in a TAC induced HF mouse model.2
A compelling example of a successful therapeutic intervention in a pressure overload-induced HF model is a study investigating the role of fucoidan in attenuating cardiac remodeling. This research utilized a transverse aortic constriction (TAC) mouse model to induce cardiac hypertrophy. The project results demonstrated that fucoidan treatment significantly attenuated the pressure overload-induced upregulation of serum Galectin-3 levels, reduced cardiac collagen deposition (fibrosis), and decreased inflammatory cell infiltration. These findings suggest that fucoidan holds promise as a therapeutic agent for preventing or delaying adverse cardiac remodeling and associated complications like fibrosis and inflammation in pressure overload-induced conditions.
References
- Farag, Ahmed et al. "A review on experimental surgical models and anesthetic protocols of heart failure in rats." Frontiers in veterinary science vol. 10 1103229. 27 Mar. 2023. Distributed under Open Access license CC BY 4.0, without modification. The image was modified by extracting and using only part of the original image. https://doi.org/10.3389/fvets.2023.1103229
- Hao, Wen-Rui et al. "Fucoidan Attenuates Cardiac Remodeling by Inhibiting Galectin-3 Secretion, Fibrosis, and Inflammation in a Mouse Model of Pressure Overload." Biomedicines vol. 12,12 2847. 14 Dec. 2024. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3390/biomedicines12122847
For Research Use Only.
