Diarrhea Modeling & Pharmacodynamics Services
Introduction
Diarrhea is a condition marked by frequent, loose, or watery stools, often due to infections, but it can also result from dietary issues, medications, or chronic diseases. Acute diarrhea, which typically lasts a few days, is commonly caused by viral, bacterial, or parasitic infections, and is frequently self-limiting. Chronic diarrhea persists for more than four weeks, often stemming from conditions like irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), or malabsorption disorders. In some cases, diarrhea may be classified based on its underlying mechanisms, such as osmotic diarrhea, which occurs when unabsorbed substances in the intestine draw water into the gut, or secretory diarrhea, where excessive fluid secretion overwhelms the intestines, often due to bacterial toxins or hormonal imbalances. Exudative diarrhea, which involves blood or pus in the stool, is often linked to infections like dysentery or conditions such as IBD. Effective management of diarrhea depends on identifying the cause and may involve rehydration therapies, antibiotics, antidiarrheal agents, or probiotics. In most cases, diarrhea is treatable, but its impact can vary greatly depending on the severity and underlying factors involved. Creative Biolabs offers a comprehensive range of rodent models to assess the efficacy of antidiarrheal treatments. These models, which include acute, chronic, and infection-induced diarrhea, are specifically designed to replicate various types of diarrhea, providing an accurate platform for drug evaluation. Our services support the development of novel therapeutics by enabling detailed assessments of drug effectiveness in treating both the symptoms and underlying causes of diarrhea.
Disease Models and Applications
Creative Biolabs offers a wide range of well-established rodent models for studying diarrhea, including models induced by infections, toxins, and other physiological factors. These models are carefully developed to mimic human diarrhea conditions, such as acute, chronic, and osmotic diarrhea, enabling a comprehensive evaluation of therapeutic candidates. Through rigorous assessment of various parameters such as stool consistency, frequency, and associated biomarkers, our models provide reliable insights into drug efficacy and safety during the preclinical phase. Our expert team works closely with you at every step, from experimental design to data interpretation, ensuring high-quality, reproducible results. To explore more about the available diarrhea models for preclinical research, please visit the links below:
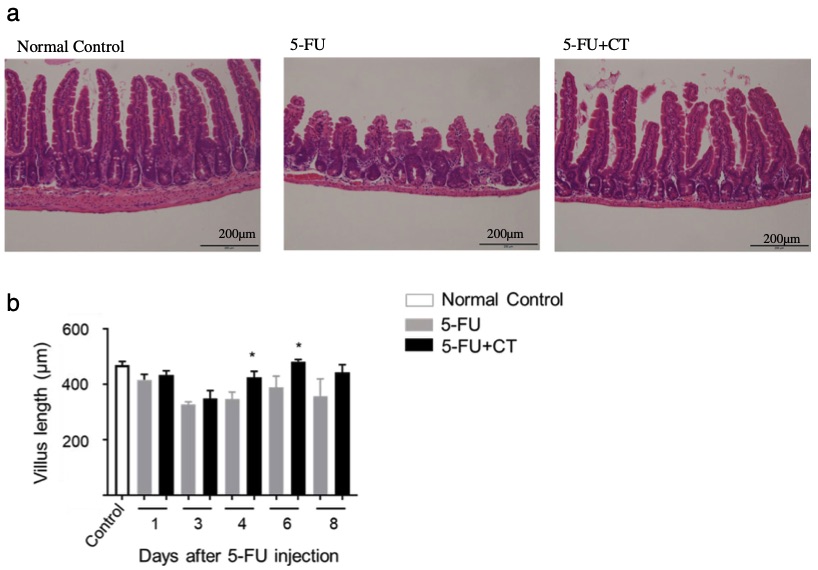 Fig. 1 The protective effects of cystine-theanine on small intestinal villi in a 5-FU-induced diarrhea model.1
Fig. 1 The protective effects of cystine-theanine on small intestinal villi in a 5-FU-induced diarrhea model.1
-
5-Fluorouracil (5-FU) induced Diarrhea Model
- Simulates: The 5-Fluorouracil (5-FU)-Induced Diarrhea Model replicates chemotherapy-induced diarrhea, a common side effect of 5-FU, which is widely used in the treatment of various cancers.
- Evaluates Drugs: This model is primarily used to assess the efficacy of drugs aimed at alleviating chemotherapy-induced diarrhea. It is suitable for testing anti-diarrheal agents, gut protectants, anti-inflammatory drugs, and probiotics. Drugs targeting gastrointestinal motility, mucosal integrity, and the inflammatory response in the gut are commonly evaluated in this model.
-
Antibiotic induced Diarrhea Model
Simulates: This model simulates the gastrointestinal issues, like diarrhea, that occur when broad-spectrum antibiotics disrupt the normal balance of gut bacteria. It effectively mimics human conditions such as antibiotic-associated diarrhea (AAD), which is often caused by an overgrowth of Clostridium difficile or other changes in the gut microbiome.
Evaluates Drugs: The model is used to test how well different drugs work to prevent or treat antibiotic-induced diarrhea. It's particularly useful for testing the anti-diarrheal agents, probiotics, and other gut microbiota modulators. Additionally, the model helps researchers screen for therapies that target Clostridium difficile infections or are designed to help restore a healthy gut microbiome.
Measurements
We offer a range of measurements to evaluate drug efficacy in rodent diarrhea models, using advanced technologies to assess multiple parameters, including but not limited to:
- General Observations: Body weight, stool consistency, stool frequency, mortality rate, and signs of dehydration or gastrointestinal distress.
- Histological Analysis: Examination of intestinal tissue to assess damage, inflammation, and structural changes such as epithelial shedding, villous atrophy, and mucosal ulceration.
- Cytokine Profiling (e.g., ELISA): Measurement of inflammatory mediators such as TNF-α, IL-6, IL-1β, and other cytokines involved in the inflammatory response, to assess the impact of treatments on intestinal inflammation.
- Hematology and Serum Biomarkers: Analysis of blood parameters including red and white blood cell counts, serum electrolytes, liver enzymes (e.g., ALT, AST), and kidney function markers (e.g., BUN, creatinine) to evaluate systemic effects and organ dysfunction.
- Gene/Protein Expression Profiling via RT-qPCR and Western Blot: Quantification of specific genes or proteins associated with inflammation, epithelial integrity, or gut motility, such as COX-2, IL-10, and tight junction proteins.
In addition to these established diarrhea models, our expertise extends to the development of custom models tailored to your specific research needs, guided by current literature and prior studies. Our scientific team is available to assist with experimental design, model selection, and data analysis, ensuring an effective and customized approach to your project at every stage.
Related Services
In addition to diarrhea models, we also offer a wide range of other preclinical models for digestive diseases to support your research needs.
Advantages
- Expertise in Preclinical Research: We provide extensive experience and scientifically validated models for drug testing, ensuring reliable results.
- Tailored Solutions: Our team offers customized approaches, from experimental design to data analysis, ensuring each project meets specific research goals.
- High-Quality Standards: We maintain rigorous quality control and adhere to international standards, ensuring the integrity of all research.
- Comprehensive Service: From initial consultations to post-study support, we provide end-to-end assistance, making the research process efficient and seamless.
- State-of-the-Art Technology: We employ the latest research technologies and techniques to provide cutting-edge solutions for drug discovery.
- Collaborative Approach: Our team of experts works closely with you to optimize research outcomes, providing personalized support throughout the project.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q1: What types of animal models do you offer for preclinical research?
A1: We offer a wide range of well-established rodent models for digestive system diseases, including those for IBD, colitis, gastric ulcers, liver disorders, and more. We also develop customized models based on your specific research needs.
-
Q2: How do you ensure the reliability of the data?
A2: We follow strict quality control protocols and use advanced technologies to ensure high-quality, reproducible results. Our team of experts works closely with you throughout the project to ensure data integrity.
-
Q3: Can you customize a model for my specific research needs?
A3: Yes, we specialize in developing custom animal models tailored to your unique research requirements. Our team will assist with experimental design, model selection, and data interpretation.
-
Q4: What drug efficacy evaluations do you offer?
A4: We provide evaluations using a variety of advanced techniques, such as general observations, immunohistochemistry, cytokine profiling, hematology analysis, and gene/protein expression analysis, among others.
-
Q5: What support do you offer during the research process?
A5: We provide end-to-end support, from experimental design to post-study data analysis. Our experienced scientists collaborate with you to optimize the research and ensure your project is successful.
Published Data
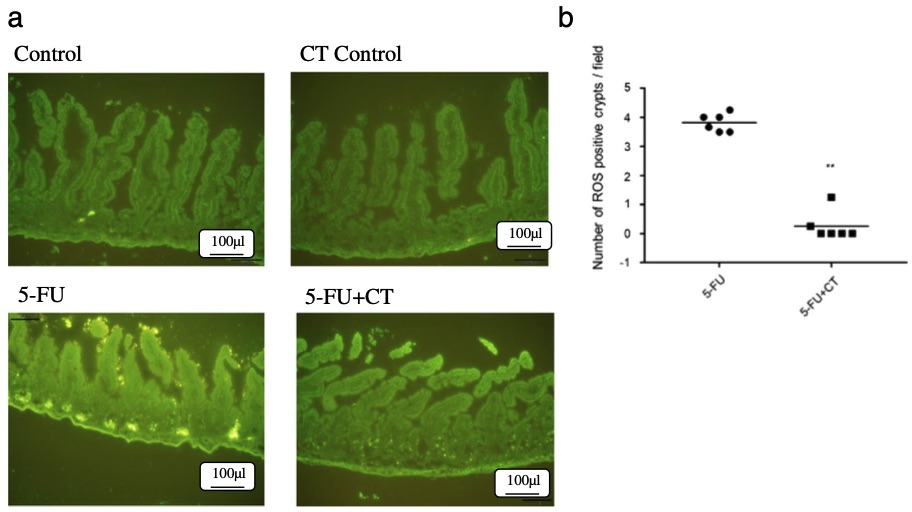 Fig. 2 Cystine-theanine suppressed 5-FU induced ROS production in the small intestine.2
Fig. 2 Cystine-theanine suppressed 5-FU induced ROS production in the small intestine.2
The inhibitory effects of cystine-theanine on mucositis and diarrhea, as well as its underlying mechanism, were investigated using a mouse model of 5-FU-induced intestinal mucositis. Panel (a) shows a fluorescence histochemical analysis of ROS in the intestinal mucosa using a ROS-specific fluorescent probe. Panel (b) presents the combined data from three experiments, displaying the number of ROS-positive crypts in the intestinal tissue.
Reference
- Yoneda, Junya et al. "Oral administration of cystine and theanine attenuates 5-fluorouracil-induced intestinal mucositis and diarrhea by suppressing both glutathione level decrease and ROS production in the small intestine of mucositis mouse model." BMC Cancer vol. 21,1 1343. 18 Dec. 2021, doi:10.1186/s12885-021-09057-z. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
