Streptozotocin (STZ) induced Type I Diabetic Peripheral Vascular Disease Modeling & Pharmacodynamics Service
Creative Biolabs offers a variety of well-established animal models to evaluate the efficacy of drugs targeting diabetes-related complications. These models are designed to simulate the disease's pathophysiology and provide a reliable platform for preclinical testing, enabling researchers to develop and assess therapeutic interventions with high precision.
Introduction
Type I diabetes is a chronic condition that results from an autoimmune destruction of insulin-producing beta cells in the pancreas, leading to lifelong insulin dependency. When left uncontrolled, Type I diabetes can cause a range of complications, including diabetic peripheral vascular disease (PVD). PVD is a condition where blood flow to the limbs, particularly the lower extremities, is restricted due to damage to blood vessels. In Type I diabetes, prolonged hyperglycemia leads to endothelial dysfunction, atherosclerosis, and thickening of the blood vessel walls, reducing blood flow. This impaired circulation hampers tissue oxygenation and nutrient delivery, which can cause pain, ulcers, and delayed wound healing in the affected limbs. The progression of diabetic PVD is often gradual, with early symptoms including intermittent claudication (leg pain while walking), cold feet, and slow-healing wounds. If left untreated, PVD can lead to more severe outcomes, including gangrene and limb amputation. In addition to the vascular damage, diabetic patients are more susceptible to infections and poor wound healing, further exacerbating the condition. Early intervention with therapies that improve circulation, reduce inflammation, and promote vascular regeneration is crucial in preventing severe complications.
Disease Models and Applications
The Streptozotocin (STZ) induced Type I Diabetic Peripheral Vascular Disease Model is a widely utilized preclinical model that simulates the vascular complications seen in diabetic patients. This model is created by administering a single dose of streptozotocin (STZ) to induce hyperglycemia and Type I diabetes in rodents. The resulting hyperglycemia leads to endothelial dysfunction, vascular damage, and reduced blood flow, which mimics the pathophysiology of diabetic peripheral vascular disease. The model is characterized by impaired angiogenesis, decreased capillary density, and poor wound healing in the extremities. It is commonly used for evaluating therapeutic strategies aimed at improving blood flow, promoting angiogenesis, and preventing limb ischemia. The advantages of this model include its reproducibility and ability to mimic human-like diabetic vascular complications. However, it has some limitations, such as the stress induced by hyperglycemia in animals and the lack of certain long-term diabetic complications present in humans.
- Simulates: The STZ induced Type I Diabetic Peripheral Vascular Disease Model simulates the vascular damage and impaired blood circulation that occurs in diabetic patients, specifically focusing on the peripheral limbs. The model mirrors diabetic induced endothelial dysfunction, reduced angiogenesis, and poor tissue perfusion, which are key factors in the progression of peripheral vascular disease.
- Evaluates Drugs: This model is utilized to evaluate drugs aimed at improving vascular health in diabetic conditions. It is particularly useful for testing therapies that target angiogenesis, endothelial function, and blood flow restoration, such as growth factors, vasodilators, anti-inflammatory drugs, and stem cell-based therapies. Additionally, it can assess drugs designed to reduce oxidative stress, improve circulation, and promote wound healing in peripheral tissues.
Measurements
We offer a variety of measurements for evaluating drug efficacy in the Streptozotocin (STZ) induced Type I Diabetic Peripheral Vascular Disease Model, utilizing advanced technologies, including but not limited to:
- General Observations: Body weight, limb perfusion.
- Doppler Ultrasound: Assessing blood flow and vascular function in the limbs to evaluate changes in perfusion.
- Immunohistochemistry: Detection of endothelial cell markers, angiogenesis, and inflammatory cells (e.g., macrophages) in vascular tissues.
- Cytokine Profiling (e.g., ELISA): Measuring inflammatory mediators such as TNF-α, IL-6, IL-1β, and VEGF to assess inflammatory responses and angiogenic activity.
- Histological Analysis: Examination of tissue morphology to assess capillary density, tissue damage, and healing in the affected limbs.
- Gene/Protein Expression Profiling via RT-qPCR and Western Blot: Analysis of key genes and proteins involved in endothelial function, angiogenesis, and inflammatory responses.
Related Services
In addition to the STZ induced diabetic peripheral vascular disease model, we also provide other diabetes-related complication models, such as those induced by high-fat diets, genetic modifications, and other chemical agents. These models are designed to evaluate a wide range of diabetic complications and therapeutic interventions.
- Streptozotocin (STZ) induced Type I Diabetic Skin Defect/Burn Model
- Streptozotocin (STZ) induced Type I Diabetic Foot Ulcer Model
- Streptozotocin (STZ) induced Type I Diabetic Cataract Model
- High-Fat Diet & Streptozotocin (STZ) induced Type II Diabetic Nephropathy Model
- db/db Type II Diabetic Nephropathy Model
Advantages
- Expert Support: Our experienced scientific team is available to assist with model selection, experimental design, and data analysis, providing personalized guidance throughout the research process.
- State-of-the-Art Technologies: We utilize advanced technologies and measurement techniques, such as Doppler ultrasound, immunohistochemistry, and cytokine profiling, to offer comprehensive data on drug efficacy and mechanisms of action.
- Reproducibility and Reliability: Our models are known for their reproducibility, providing consistent and reliable results across studies to support therapeutic development and regulatory submissions.
- Innovative Solutions: We continuously develop and refine our models to stay at the forefront of diabetes research, offering cutting-edge solutions to meet the evolving needs of the scientific community.
- Comprehensive Services: From model development to data analysis, we offer a full range of services, ensuring seamless support throughout your project and helping you achieve the best possible outcomes in diabetes research.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q: What is the STZ induced Type I Diabetic Peripheral Vascular Disease Model?
A: The STZ induced model is used to simulate diabetic vascular complications in rodents by inducing Type I diabetes through streptozotocin, leading to impaired blood flow and peripheral vascular disease.
-
Q: What drugs can be evaluated using this model?
A: This model is ideal for evaluating drugs aimed at improving angiogenesis, endothelial function, blood circulation, and wound healing, such as vasodilators, growth factors, and anti-inflammatory agents.
-
Q: How is blood flow measured in this model?
A: Blood flow is typically assessed using Doppler ultrasound, allowing for a real-time measurement of limb perfusion and vascular function.
-
Q: Can this model be customized?
A: Yes, we offer the ability to customize the model, including variations in the diabetic induction method, severity of vascular damage, and treatment protocols to suit your specific research needs.
-
Q: What measurements are included in the evaluation process?
A: We provide a comprehensive range of measurements, including general observations, Doppler ultrasound, immunohistochemistry, cytokine profiling, histological analysis, and gene/protein expression profiling.
Published Data
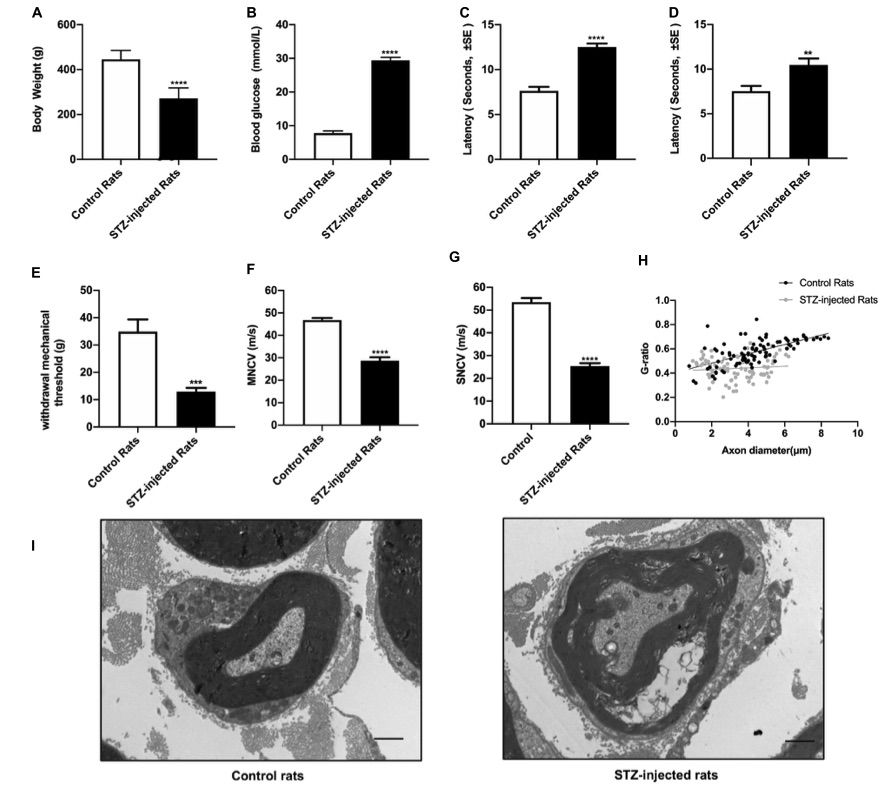 Fig. 1 Evaluation of streptozotocin (STZ)-injected diabetic peripheral neuropathy (DPN) rat models.1
Fig. 1 Evaluation of streptozotocin (STZ)-injected diabetic peripheral neuropathy (DPN) rat models.1
Eight weeks following the STZ injection, a series of assessments was conducted to evaluate the signs and symptoms of diabetic peripheral neuropathy (DPN), including body weight, blood glucose levels, thermal and mechanical stimuli thresholds, nerve conduction velocity, and morphological changes in the sciatic nerve. The results indicated that, compared to control groups, the STZ-treated rats exhibited increased non-fasting blood glucose levels, consistent with the diagnostic criteria for diabetes (Figure 1A), while their body weight decreased (Figure 1B), confirming the successful establishment of diabetic rat models. Sensory function of the sciatic nerve was evaluated through the tail flick test, hot plate test, and von Frey hair test. In addition, both motor and sensory nerve conduction velocities of the sciatic nerve were measured. The data revealed nerve conduction slowing (Figures 1F, G), an elevated mechanical threshold (Figure 1E), and a reduced thermal threshold (Figures 1C, D), indicative of sciatic nerve damage caused by DPN. Morphological analysis further revealed that the g-ratio of myelinated fibers was significantly altered by DPN (Figure 1H). Histological examination of nerve fibers from diabetic rats showed swelling and loss of myelinated fibers (Figure 1I).
Reference
- Wang, Cheng et al. "The Construction and Analysis of lncRNA-miRNA-mRNA Competing Endogenous RNA Network of Schwann Cells in Diabetic Peripheral Neuropathy." Frontiers in bioengineering and biotechnology vol. 8 490. 25 May. 2020, DOI:10.3389/fbioe.2020.00490. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
