IL-10-/- Spontaneous Inflammatory Bowel Disease (IBD) Modeling & Pharmacodynamics Service
Introduction
Inflammatory Bowel Disease (IBD) refers to a group of chronic, relapsing inflammatory disorders affecting the gastrointestinal tract, with the two main types being Crohn's disease (CD) and ulcerative colitis (UC). Crohn's disease can involve any part of the digestive tract, from the mouth to the anus, and is characterized by transmural inflammation, often with segmental lesions and complications such as strictures, fistulas, and granulomas. Ulcerative colitis, on the other hand, is limited to the colon and rectum, with continuous mucosal inflammation that typically starts from the rectum and extends proximally. The pathogenesis of IBD involves both innate and adaptive immune mechanisms, dysregulation of cytokines such as TNF-α, IL-6, IL-23, and disruptions in intestinal barrier integrity. While current therapies—including corticosteroids, 5-aminosalicylic acid (5-ASA), immunosuppressants, biologics, and small molecule inhibitors—help manage inflammation and maintain remission, many patients experience treatment failure or relapse, highlighting the urgent need for more effective and targeted therapies. Creative Biolabs offers a full suite of validated rodent models for IBD research, including DSS-, TNBS-, DNBS-, oxazolone-, acetic acid-, and anti-CD40 antibody-induced colitis models. These models replicate various aspects of human IBD pathophysiology and are supported by comprehensive analytical tools, including histopathology, cytokine assays, immune cell profiling, and gene/protein expression analysis. Our expert team provides end-to-end support to accelerate your IBD drug discovery and development programs.
Disease Models and Applications
The IL-10 knockout (KO) mouse spontaneous inflammatory bowel disease (IBD) model is a genetically engineered model in which the interleukin-10 gene is deleted, resulting in the loss of a key anti-inflammatory cytokine. These mice develop spontaneous chronic colitis under specific pathogen-free (SPF) or conventional housing conditions, typically starting from 6 to 10 weeks of age. The disease closely resembles human Crohn’s disease, featuring transmural inflammation, epithelial hyperplasia, crypt abscesses, and a Th1-skewed cytokine profile. This model is particularly valuable for studying the role of immune regulation and gut microbiota in IBD pathogenesis. It does not require chemical or surgical induction, allowing for long-term studies of disease development and progression. However, disease onset and severity can vary depending on environmental factors such as microbiota composition and housing conditions, which may impact reproducibility. Despite this, the IL-10 KO model is widely used for evaluating immunomodulatory therapies and investigating host-microbiome interactions in chronic IBD.
- Simulates: The IL-10 KO Mouse Spontaneous Inflammatory Bowel Disease (IBD) Model simulates chronic inflammatory bowel conditions, particularly Crohn’s disease. It replicates key features such as transmural inflammation, epithelial hyperplasia, crypt abscesses, and elevated Th1/Th17 cytokine responses, making it ideal for studying immune dysregulation and microbiota-driven pathogenesis.
- Evaluates Drugs: This model is used to evaluate immunomodulatory agents, including anti-inflammatory drugs, cytokine inhibitors (e.g., anti-IL-12/23, anti-TNF-α), probiotics, microbiota-targeted therapies, and experimental biologics aimed at restoring immune tolerance and mucosal homeostasis in chronic IBD.
Measurements
We offer a variety of measurements for evaluating drug efficacy in the IL-10 knockout (KO) mouse spontaneous inflammatory bowel disease (IBD) model, utilizing a range of advanced technologies, including but not limited to:
- General observations: Body weight changes, stool consistency, diarrhea frequency, presence of blood in feces, and overall mortality rate.
- Macroscopic assessment: Colon length, colon wall thickening, and ulceration scoring to assess disease severity.
- Histopathological analysis: Hematoxylin and eosin (H&E) staining to examine colonic inflammation, epithelial damage, crypt loss, and immune cell infiltration.
- Cytokine profiling (e.g., ELISA): Quantification of pro-inflammatory cytokines, including TNF-α, IL-6, IL-1β, and IFN-γ, which are elevated during inflammation.
- Myeloperoxidase (MPO) activity assay: Measurement of neutrophil infiltration as a marker of inflammation.
- Gene/protein expression profiling via RT-qPCR and Western blot: Evaluation of inflammatory mediators, immune checkpoint molecules, and mucosal barrier proteins to assess the underlying immune response and intestinal integrity.
In addition to the established IL-10 KO IBD model, our expertise extends to the development of customized models based on specific research needs. Our scientific team is available to assist in experimental design, model selection, and data analysis, ensuring a tailored approach to your research at every stage.
Related Services
In addition to the IL-10 KO spontaneous IBD model, we also offer chemically and immunologically induced colitis models. These models represent diverse pathological mechanisms of IBD.
- TNBS/DNBS induced Colitis Model
- DSS induced Colitis Model
- Indomethacin induced Small Intestinal Inflammatory Model
- OXA induced Colitis Model
- Acetic Acid induced IBD Model
- Anti-CD40 Ab induced IBD Model
- CD4+CD45RBhi T Cells induced IBD Model
Advantages
1. Customized Solutions: Our scientific team offers tailored solutions, developing and adapting models based on specific therapeutic targets, experimental conditions, and research objectives.
2. Advanced Analytical Techniques: We use cutting-edge technologies, such as cytokine profiling, gene/protein expression analysis, histopathology, and immune cell infiltration studies, ensuring reliable and reproducible results.
3. Expert Support: With extensive experience in preclinical research, our team provides full support from study design and model selection to data analysis and reporting.
4. Quality and Reproducibility: We adhere to strict quality control protocols to ensure consistent, high-quality data across studies, facilitating the translation of research findings into effective therapeutic interventions.
5. Efficient, End-to-End Service: From initial consultation to final report, we offer seamless service, optimizing your research timeline while delivering precise, actionable insights.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q1: What species and strains are used in your models?
A1: We commonly use C57BL/6, BALB/c mice, and Wistar or Sprague Dawley rats, depending on the model type and study design. Genetically modified strains like IL-10 KO or Rag1 KO are also available.
-
Q2: Do you offer support with study design and data interpretation?
A2: Absolutely. Our experienced team provides full support throughout your project, from protocol design and model selection to experimental execution, data analysis, and final reporting.
-
Q3: How do you ensure data quality and reproducibility?
A3: All experiments are conducted under standardized protocols with rigorous quality control. We ensure reproducibility through validated procedures and consistent animal care standards.
-
Q4: Can you assist with IND-enabling studies or regulatory documentation?
A4: Yes, we can generate high-quality preclinical data suitable for regulatory submissions and provide supporting documentation as needed for IND or other regulatory filings.
Published Data
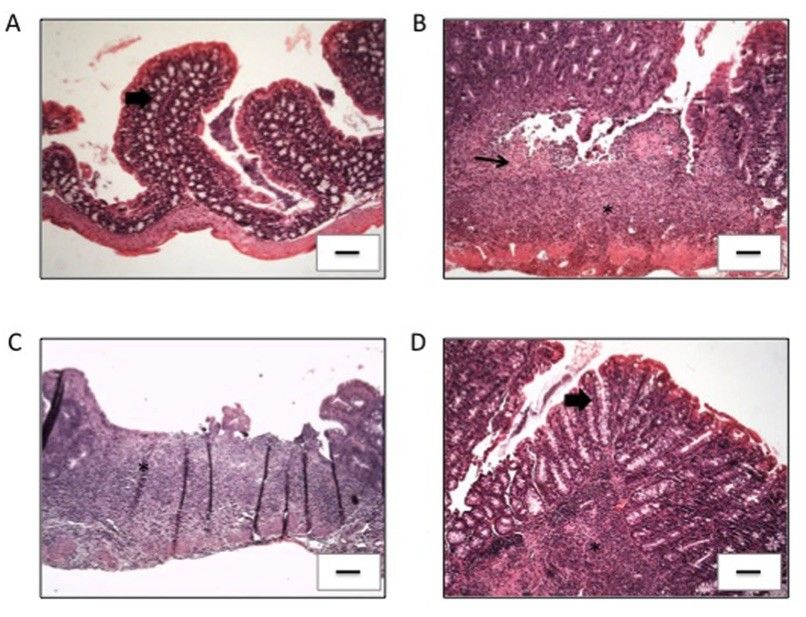 Fig. 1 Histopathology of changes in the AC of all experimental groups.1
Fig. 1 Histopathology of changes in the AC of all experimental groups.1
This study aimed to evaluate the therapeutic effect of a candidate treatment in IL-10-deficient (IL-10⁻/⁻) mice, which spontaneously develop chronic intestinal inflammation resembling Crohn's disease (CD) in humans. Hematoxylin and eosin (H&E) staining of the ascending colon (AC) in the control group revealed normal histological architecture, including intact mucosa, thin submucosal and serosal layers, appropriate muscular layer thickness, and a normal distribution of goblet cells, with no signs of degeneration, inflammation, or abnormal cell proliferation (Fig. 1a). In contrast, all IL-10⁻/⁻ mice except those treated with pValac:il-10 showed similar macroscopic and histological damage in the AC, including compromised tissue structure, moderate mucosal inflammatory infiltrates, presence of mononuclear cells, epithelial erosion, and goblet cell reduction. Inflammation and edema were also observed in the submucosal layer, while the muscular and serosal layers remained unaffected (Fig. 1b, c). Mice in the pValac:il-10 group showed marked histological improvement, with only mild inflammation in less than 40% of animals. Most had no evidence of erosion, goblet cell depletion, or submucosal inflammatory infiltration (Fig. 1d), indicating a protective and therapeutic effect in this spontaneous IBD model.
Reference
- Zurita-Turk, Meritxell et al. "Attenuation of intestinal inflammation in IL-10 deficient mice by a plasmid carrying Lactococcus lactis strain." BMC Biotechnology vol. 20,1 38. 23 Jul. 2020, doi:10.1186/s12896-020-00631-0. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
