Lysophosphatidic Acid (LPA) induced Acute Kidney Injury Modeling & Pharmacodynamics Service
Creative Biolabs offers a comprehensive range of well-established and robust animal models designed to evaluate drug efficacy for the treatment of AKI. By using these models, researchers can assess the potential of novel therapeutic candidates, investigate underlying mechanisms of kidney injury, and evaluate the safety and effectiveness of interventions aimed at mitigating renal damage. These models provide a crucial foundation for advancing drug development and improving clinical outcomes for patients suffering from AKI.
Introduction
Acute Kidney Injury (AKI) refers to a sudden loss of kidney function, characterized by an abrupt increase in serum creatinine or a decrease in urine output. It can occur because of a variety of conditions, including ischemia, nephrotoxic drugs, infections, or systemic diseases. AKI is classified into three main types: prerenal, intrinsic, and postrenal, each corresponding to different causes of kidney dysfunction. The condition is associated with a high mortality rate, especially when progression to chronic kidney disease (CKD) occurs. The pathophysiology of AKI involves complex interactions between hemodynamic changes, cellular injury, inflammation, and oxidative stress, which ultimately lead to tubular dysfunction, apoptosis, and fibrosis. AKI can be caused by a variety of agents, including nephrotoxins, ischemic injury, and systemic inflammation. In many cases, it is reversible if treated promptly, but the inability to predict or prevent its onset remains a significant challenge. This condition is often seen in critically ill patients, particularly those in intensive care units, and is a major cause of morbidity and mortality worldwide.
Lysophosphatidic acid (LPA)-Induced Acute Kidney Injury Model
The Lysophosphatidic acid (LPA)-Induced Acute Kidney Injury Model is a valuable tool for studying the pathophysiology of AKI, particularly in understanding the mechanisms behind tubular injury and inflammation. LPA, a bioactive lipid, induces a cascade of cellular events, including oxidative stress, inflammation, and fibrosis, making it a suitable model for drug screening and mechanism elucidation. In this model, LPA is administered to rodents, leading to kidney damage like human AKI, including tubular necrosis and inflammatory cell infiltration. The advantage of this model lies in its ability to closely mimic both the acute and chronic phases of kidney injury, allowing for the assessment of therapeutic agents aimed at reducing inflammation, preventing fibrosis, or promoting renal recovery. While this model offers high relevance to AKI research, its limitation lies in the variability in the severity of kidney injury, which may require optimization depending on the research focus. The LPA model is commonly used in studies investigating targeted therapies that aim to modulate cellular stress responses, inflammation, and fibrosis in the kidney.
- Simulates: This model simulates various kidney injuries associated with acute renal stress, providing insights into the role of lipids in renal pathophysiology. It mimics inflammation, oxidative stress, and nephron damage, crucial for AKI studies.
- Evaluates Drugs: It is ideal for evaluating anti-inflammatory, anti-fibrotic, and nephroprotective drugs. The model also helps assess the efficacy of agents that modulate lipid metabolism, oxidative stress, and cellular injury in kidney cells.
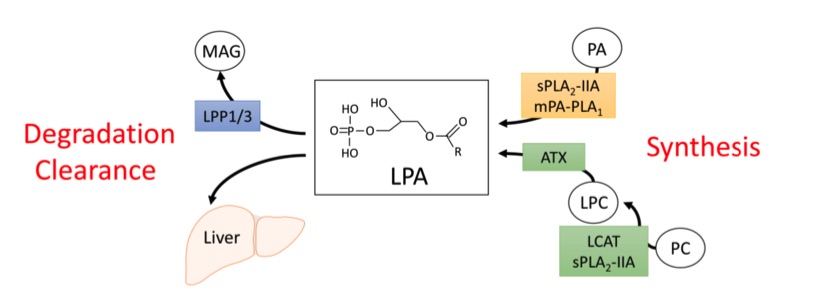 Fig. 1 Metabolism of circulating lysophosphatidic acid (LPA).1,3
Fig. 1 Metabolism of circulating lysophosphatidic acid (LPA).1,3
Evaluation Platform
- Animals: Mouse, Rat.
-
Measurements
We offer a range of measurements to evaluate drug efficacy in LPA-induced AKI models, utilizing the latest in advanced technology:- General Observations: Body weight, kidney function (creatinine, BUN levels), mortality rate, and clinical scoring.
- Histopathology: Tissue staining (H&E, PAS) to assess kidney damage, tubular dilation, necrosis, and inflammatory cell infiltration.
- Cytokine Profiling (ELISA): Measurement of pro-inflammatory markers such as IL-6, TNF-α, and IL-1β.
- Oxidative Stress Assays: Detection of ROS levels and oxidative damage using specific biomarkers (e.g., MDA, GSH).
- Gene/Protein Expression Profiling: RT-PCR and Western blot analysis to evaluate markers of fibrosis (e.g., TGF-β, collagen) and inflammation (e.g., NF-kB, MCP-1).
Our services also include custom analysis based on your research needs, ensuring an effective approach from experimental design to data interpretation.
Related Services
In addition to the LPA-induced AKI model, we offer several other AKI models induced by agents like cisplatin, glycerol, or folic acid. These models allow for comprehensive testing across various mechanisms of kidney injury.
Our advantages
- Tailored Solutions: We offer customized experimental designs to meet your specific research goals.
- Advanced Technology: Access to cutting-edge tools for precise data collection and analysis.
- Expert Support: Our scientific team assists with model selection, experimental design, and data interpretation.
- Reproducibility: We guarantee reliable and reproducible results in every study.
- Comprehensive Models: A wide range of AKI models to assess various drug candidates targeting different aspects of kidney injury.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What are the benefits of using the LPA model for AKI research?
The LPA model mimics both acute and chronic aspects of kidney injury, making it a versatile tool for testing drugs that target inflammation and fibrosis.
-
2. Can I use the LPA model to test nephroprotective drugs?
Yes, this model is ideal for evaluating drugs aimed at reducing inflammation and preventing kidney damage, particularly those with anti-inflammatory or anti-fibrotic properties.
-
3. How long does it take to establish the model?
The model typically requires 2-3 days for the induction of kidney injury after LPA administration, with assessment continuing for several weeks depending on the treatment protocol.
Published Data
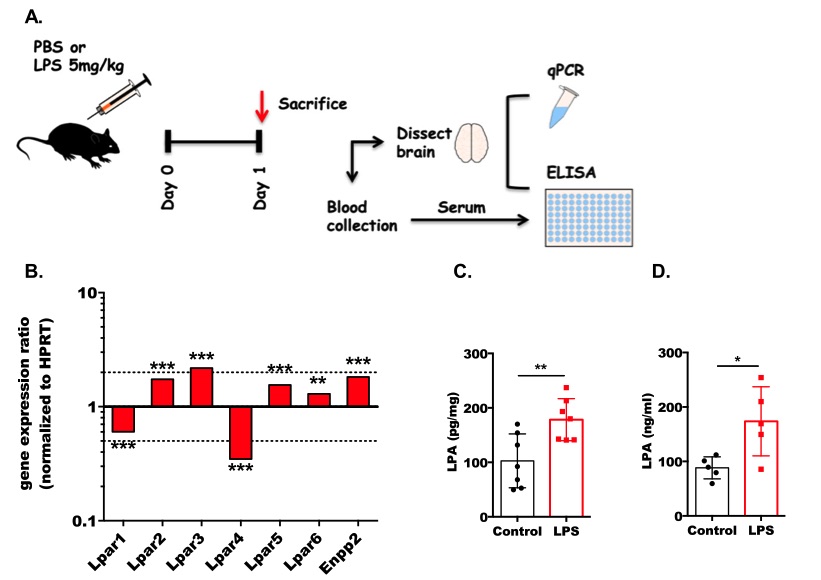 Fig 2. Acute inflammation differentially regulates the expression of LPARs and ENPP2 and increases LPA concentrations in mouse brain.2,3
Fig 2. Acute inflammation differentially regulates the expression of LPARs and ENPP2 and increases LPA concentrations in mouse brain.2,3
In this experiment, C57BL/6 J mice were administered either a single high dose (100 μg/20 g body weight) or four consecutive daily low doses (28 μg/20 g body weight) of LPS. The treatment and experimental protocols are depicted in Fig. 2a. Following the induction of septic conditions, the expression of LPAR1 and LPAR4 was significantly downregulated, while LPAR2, LPAR3, LPAR5, and LPAR6, along with ENPP2, were transcriptionally upregulated (Fig. 2b). Consistent with the increased expression of ENPP2, LPA concentrations in the brain were significantly elevated in response to LPS treatment (Fig. 2c). Additionally, serum LPA levels were also significantly increased following endotoxin exposure (Fig. 2d).
References
- D'Souza, Kenneth et al. "Lysophosphatidic Acid Signaling in Obesity and Insulin Resistance." Nutrients vol. 10,4 399. 23 Mar. 2018. https://doi.org/10.3390/nu10040399
- Plastira, Ioanna et al. "MAPK signaling determines lysophosphatidic acid (LPA)-induced inflammation in microglia." Journal of Neuroinflammation vol. 17,1 127. 23 Apr. 2020. https://doi.org/10.1186/s12974-020-01809-1
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
