Acetic Acid induced Inflammatory Bowel Disease (IBD) Modeling & Pharmacodynamics Service
Introduction
Inflammatory Bowel Disease (IBD) is a group of chronic, relapsing inflammatory conditions of the gastrointestinal (GI) tract, primarily including Crohn's disease (CD) and ulcerative colitis (UC). These diseases are characterized by aberrant immune responses to intestinal microbiota in genetically predisposed individuals, leading to persistent intestinal inflammation and tissue damage. Crohn's disease can affect any part of the GI tract from the mouth to the anus, with inflammation often penetrating the entire thickness of the bowel wall. In contrast, ulcerative colitis is typically limited to the colon and rectum, with inflammation confined to the mucosal layer. Common symptoms of IBD include abdominal pain, diarrhea, rectal bleeding, weight loss, and fatigue. The disease course is marked by periods of remission and exacerbation, which significantly impact patients' quality of life. The exact cause of IBD remains unclear, but it is believed to involve a combination of genetic susceptibility, environmental triggers, dysbiosis of gut microbiota, and immune system dysregulation. Current therapeutic strategies include anti-inflammatory drugs (e.g., 5-aminosalicylic acid), corticosteroids, immunosuppressants, biologics (e.g., TNF-α inhibitors), and small-molecule inhibitors (e.g., JAK inhibitors). Despite advances in treatment, many patients experience inadequate response or develop resistance over time, highlighting the need for continued preclinical research and drug development. Creative Biolabs offers a wide range of well-established rodent models for evaluating the efficacy of drug candidates targeting IBD, including TNBS/DNBS-, DSS-, oxazolone-, and acetic acid-induced colitis models. Our services include model establishment, dosing, clinical scoring, histopathological analysis, cytokine profiling, and more. With advanced technologies and expert guidance, we ensure comprehensive and reliable preclinical data to support your IBD research and development pipeline.
Disease Models and Applications
The Acetic Acid-Induced Inflammatory Bowel Disease (IBD) Model is a widely used chemical model for simulating ulcerative colitis in rodents. It is established by administering a diluted solution of acetic acid, typically 3–5%, directly into the colon via rectal instillation under mild anesthesia. This leads to a rapid onset of mucosal damage characterized by epithelial erosion, crypt distortion, infiltration of inflammatory cells, and ulceration, resembling human ulcerative colitis. The model is simple, cost-effective, and reproducible, making it suitable for short-term studies and initial screening of anti-inflammatory compounds. It allows for evaluation of clinical signs such as body weight loss, stool consistency, and colon length, along with histological and biochemical analyses. However, a major limitation of the acetic acid model is its acute and self-limiting nature, which does not fully recapitulate the chronicity and immune-mediated mechanisms seen in human IBD. Additionally, variability in severity can occur depending on acid concentration, exposure time, and animal strain. Despite these limitations, the model remains a valuable tool for assessing mucosal healing and testing novel therapeutic agents targeting early-stage inflammation.
- Simulates: The Acetic Acid-Induced Inflammatory Bowel Disease (IBD) Model simulates acute ulcerative colitis, particularly the early inflammatory phase of the disease. It mimics key pathological features such as mucosal ulceration, epithelial damage, crypt loss, and neutrophil infiltration, resembling the acute flares seen in human UC.
- Evaluates Drugs: This model is commonly used to evaluate the efficacy of anti-inflammatory agents (e.g., 5-aminosalicylic acid, corticosteroids), mucosal protectants, antioxidants, and experimental compounds targeting epithelial repair and inflammation resolution in acute colitis.
Measurements
We offer a variety of measurements for evaluating drug efficacy in the acetic acid-induced inflammatory bowel disease (IBD) model, utilizing a range of advanced technologies and validated indicators, including but not limited to:
- General observations: Body weight changes, stool consistency, fecal occult blood, and survival rate.
- Macroscopic assessment: Colon length, wall thickening, ulcer index, and edema scoring.
- Histopathological analysis: Hematoxylin and eosin (H&E) staining for epithelial damage, crypt loss, mucosal ulceration, and inflammatory cell infiltration.
- Myeloperoxidase (MPO) activity assay: Quantification of neutrophil infiltration in colon tissues.
- Cytokine profiling (e.g., ELISA): Measurement of inflammatory mediators such as TNF-α, IL-1β, and IL-6.
- Oxidative stress markers: Levels of malondialdehyde (MDA), glutathione (GSH), and superoxide dismutase (SOD) in colon tissue.
- Gene and protein expression: RT-qPCR and Western blotting for inflammatory pathway markers, tight junction proteins, and apoptotic factors.
In addition to this well-established colitis model, we also develop customized animal models based on literature and project-specific requirements. Our experienced team provides full support from experimental design to comprehensive data analysis, ensuring reliable and tailored preclinical research outcomes.
Related Services
Besides the Acetic Acid-Induced inflammatory bowel disease (IBD) model, we provide a variety of other colitis models designed to explore different immunopathological mechanisms and aspects of intestinal inflammation, including:
- TNBS/DNBS induced Colitis Model
- DSS induced Colitis Model
- Indomethacin induced Small Intestinal Inflammatory Model
- OXA induced Colitis Model
- Anti-CD40 Ab induced IBD Model
- IL-10 KO Mouse Spontaneous IBD Model
- CD4+CD45RBhi T Cells induced IBD Model
Advantages
- Extensive Model Portfolio: We offer a comprehensive range of well-established rodent models for digestive system diseases, including multiple types of colitis, gastric ulcers, and intestinal inflammation, to meet diverse research needs.
- Customized Solutions: Our scientific team can tailor experimental designs and develop novel animal models based on specific project goals, literature references, or client requirements.
- Advanced Evaluation Techniques: We utilize cutting-edge technologies such as ELISA, RT-qPCR, Western blotting, immunohistochemistry, and histopathology to ensure robust and accurate drug efficacy assessments.
- Experienced Research Team: With years of expertise in preclinical drug evaluation, our team provides professional support in model selection, protocol optimization, and data interpretation.
- High Reproducibility and Quality Control: All experiments are conducted under standardized protocols and rigorous quality management to ensure consistency and reliability of results.
- One-Stop Service: From initial consultation to final report delivery, we offer seamless end-to-end support, ensuring efficient project execution and timely communication.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q1: What types of colitis models do you offer?
A1: We provide a variety of rodent colitis models, including TNBS-, DNBS-, DSS-, OXA-, and adoptive T cell transfer-induced models, each tailored to mimic different pathological and immunological features of human inflammatory bowel diseases.
-
Q2: Can you customize a colitis model based on our research needs?
A2: Yes. Our team can adjust parameters such as induction method, dosage, duration, and evaluation endpoints, or even develop new models based on literature and client-specific objectives.
-
Q3: What kinds of readouts and analyses do you provide?
A3: We offer a full suite of evaluations, including clinical scoring (DAI), colon length measurement, histopathology, cytokine profiling (ELISA), gene/protein expression (qPCR, Western blot), MPO activity, and immune cell analysis (IHC, flow cytometry).
-
Q4: How are animal welfare and ethical standards maintained?
A4: All studies are conducted in compliance with international guidelines for the ethical use of animals in research. Our animal care practices follow strict protocols to ensure humane treatment.
-
Q5: What species and strains are used in your colitis models?
A5: We commonly use C57BL/6 and BALB/c mice, as well as Sprague Dawley and Wistar rats, depending on the model type and experimental requirements.
-
Q6: Can you assist with study design and data interpretation?
A6: Absolutely. Our experienced scientists provide support at every stage, from experimental planning and model selection to data analysis and reporting.
Published Data
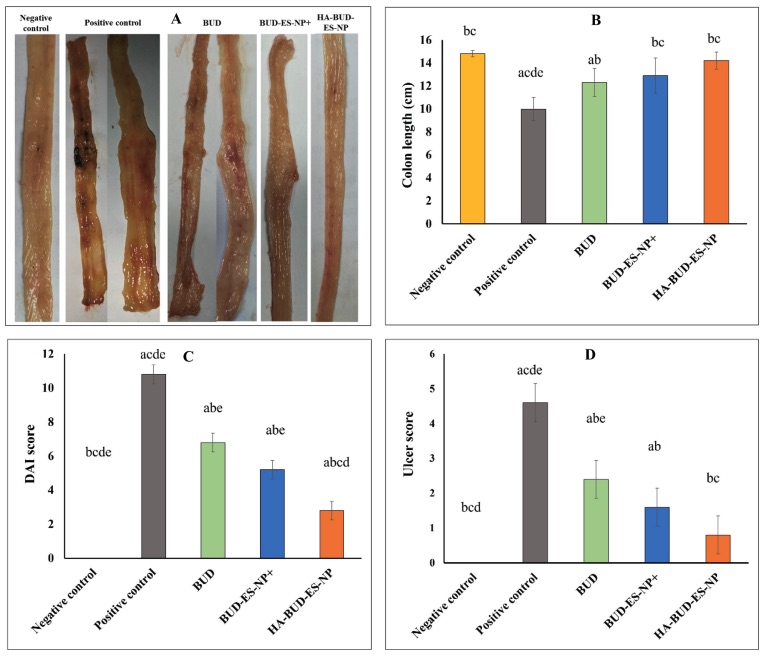 Fig. 1 Effect on colon length, DAI, and macroscopic ulcer score was evaluated in the acetic acid-induced IBD model.1
Fig. 1 Effect on colon length, DAI, and macroscopic ulcer score was evaluated in the acetic acid-induced IBD model.1
This study investigated the therapeutic effects of HA-BUD-ES-NPs in an acetic acid-induced IBD model. IBD induction resulted in a significant decrease in colon length and a marked increase in disease activity index (DAI) and macroscopic ulcer score compared to the negative control group (Fig. 7A–D). Treatment with BUD, BUD-ES-NP+, or HA-BUD-ES-NP significantly improved these parameters versus the positive control. Notably, HA-BUD-ES-NP showed the most effective recovery, with colon length and ulcer score approaching those of the negative control group, indicating substantial mucosal healing and inflammation reduction.
Reference
- Seoudi, Shaymaa S et al. "Targeted delivery of budesonide in acetic acid induced colitis: impact on miR-21 and E-cadherin expression." Drug Delivery and Translational Research vol. 13,11 (2023): 2930-2947. doi:10.1007/s13346-023-01363-2. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
