Indomethacin induced Refractory Gastric Ulcer Modeling & Pharmacodynamics Service
Introduction
Indomethacin-induced refractory gastric ulcers are a specific type of gastric ulcer commonly induced using nonsteroidal anti-inflammatory drugs (NSAIDs). Indomethacin, a widely used NSAID, can cause gastric mucosal injury by inhibiting prostaglandin synthesis, leading to reduced mucus production, impaired blood flow, and increased acid secretion. These factors combine to disrupt the protective mucosal layer, resulting in ulcer formation. This model is particularly valuable for studying NSAID-induced ulcers and the underlying mechanisms of gastric mucosal injury. Symptoms include pain, nausea, and gastrointestinal bleeding, with potential complications such as perforation and hemorrhage. Given the prevalence of NSAID use in both clinical and over-the-counter settings, this model is crucial for evaluating new therapeutic strategies for managing NSAID-induced gastric damage.
Creative Biolabs offers an advanced and well-established indomethacin-induced gastric ulcer model to assess the efficacy of potential treatments. The model is useful for evaluating the efficacy of drugs aimed at managing refractory gastric ulcers and improving mucosal healing.
Disease Models and Applications
The indomethacin-induced rodent gastric ulcer model is designed to replicate the pathophysiology of NSAID-induced gastric ulcers. Indomethacin is orally administered to rodents, which leads to gastric mucosal damage through the inhibition of cyclooxygenase (COX) enzymes, reducing protective prostaglandins in the stomach lining. This model mimics the chronic and severe nature of NSAID-induced ulcers, particularly in cases where the ulceration is refractory to typical treatments. The model provides an opportunity to evaluate therapies targeting COX enzymes, prostaglandin analogs, or other pharmacological agents aimed at promoting gastric mucosal protection and repair. Its primary advantage is the ability to study ulcers resistant to conventional treatments, including proton pump inhibitors (PPIs) and H2-receptor antagonists. However, the model's limitation lies in its focus on NSAID-induced ulceration, which may not fully mimic ulcers caused by other factors like H. pylori infection or alcohol.
- Simulates: This model replicates the chronic and refractory nature of NSAID-induced gastric ulceration, providing insight into the mechanisms of gastric mucosal injury and repair in the presence of NSAIDs.
- Evaluates Drugs: This model is used to evaluate drugs aimed at treating refractory gastric ulcers, including COX inhibitors, prostaglandin analogs, mucosal protectants, and anti-inflammatory drugs.
Measurements
We offer a comprehensive range of measurements for evaluating drug efficacy in the indomethacin-induced rodent gastric ulcer model, utilizing advanced techniques, including but not limited to:
- General Observations: Body weight, ulcer area measurement, mortality rate, food/water intake, and signs of gastric distress.
- Endoscopic Evaluation: Visualization and measurement of ulcer size and severity in the gastric mucosa.
- Histopathological Analysis: Assessment of gastric tissue damage, including mucosal erosion, inflammation, and hemorrhage through hematoxylin and eosin (H&E) staining.
- Cytokine Profiling (e.g., ELISA): Expression levels of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β, which are elevated in ulceration.
- Oxidative Stress Markers: Measurement of reactive oxygen species (ROS) and antioxidant enzymes like superoxide dismutase (SOD), catalase, and glutathione peroxidase.
- Gene/Protein Expression Profiling via RT qPCR and Western Blot Techniques: Expression of gastric injury markers, such as COX-2, MMPs, and mucin proteins, to assess the degree of tissue repair and inflammation.
In addition to the established model, our scientific team can also develop customized rodent models based on literature and prior studies to tailor your research to specific needs.
Related Services
In addition to the Indomethacin-Induced refractory gastric ulcer model, we also provide other methods to induce gastric ulcers, offering a broader range of research options.
Advantages
- Expertise and Experience: With years of specialized knowledge in preclinical models, we provide scientifically rigorous and reliable models for refractory gastric ulcer research.
- Comprehensive Services: From experimental design to data analysis, we offer full support, ensuring that the study aligns with your research goals.
- Cutting-Edge Technologies: Our advanced technologies, including cytokine profiling, histopathological analysis, and gene/protein expression profiling, guarantee precise and meaningful results.
- Customized Models: In addition to the established model, we can develop novel, customized models tailored to your specific research objectives.
- Collaborative Approach: Our scientific team collaborates closely with you to ensure that each phase of your study aligns with your overall research strategy.
- Reliable Results: Our models are standardized, ensuring reproducible and consistent data, which is essential for reliable drug evaluation and preclinical studies.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q1: How long does it take to conduct a study using the acetic acid & indomethacin-induced refractory gastric ulcer model?
A1: The study duration can vary based on the specific objectives and scope of your project. Typically, preclinical studies using this model take from a few weeks to a couple of months, including the time needed for data collection and analysis.
-
Q2: What support do you provide during the study?
A2: Our team offers full support, including experimental design, model selection, data collection, analysis, and interpretation, ensuring a smooth and effective research process from start to finish.
-
Q3: Do you offer other disease models besides refractory gastric ulcers?
A3: Yes, we provide a wide range of rodent disease models, including those for liver disease, cancer, atherosclerosis, kidney fibrosis, and many more, tailored to your specific research needs.
-
Q4: How do I get started with your services?
A4: To begin, simply reach out to us to discuss your research goals. Our team will help you select the most suitable model, design your study, and establish a timeline to launch your project.
-
Q5: Are your models validated for publication?
A5: Yes, our models are rigorously validated based on well-established scientific protocols and are suitable for generating publishable results. We ensure the highest standards of quality and reproducibility in all our models.
Published Data
he aim of the present study was to investigate the potential gastroprotective effects of irbesartan in an indomethacin-induced gastric injury model in rats, with the objective of identifying a single drug capable of concurrently managing hypertension and gastric injury. Indomethacin administration resulted in significant gastric damage, characterized by focal coagulative necrosis of the gastric mucosa, submucosal edema, congestion of submucosal blood vessels, and extensive infiltration of inflammatory cells in both the mucosal and submucosal layers (Fig. 1A). These pathological alterations were reflected in an increased ulcer index (Fig. 1B), pathological score (Fig. 1C), and elevated gastric acidity (Fig. 1). Pretreatment with ranitidine and irbesartan notably mitigated the indomethacin-induced gastric lesions, leading to a reduction in the pathological score, ulcer index, and gastric acidity. The preventive indices for rats pretreated with ranitidine and irbesartan were 58.7% and 41.6%, respectively.
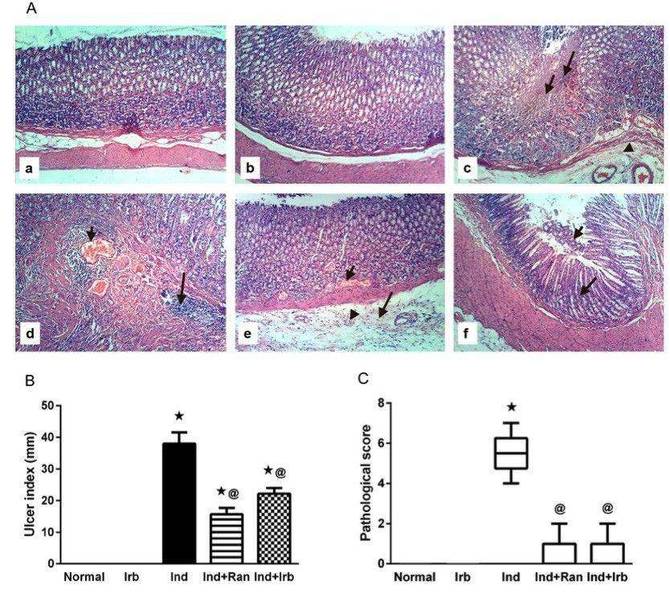 Fig. 1 Effect of irbesartan on gastric mucosal damage induced by indomethacin in rats.1
Fig. 1 Effect of irbesartan on gastric mucosal damage induced by indomethacin in rats.1
Reference
- Shahin, Nancy N et al. "A Novel Role of Irbesartan in Gastroprotection against Indomethacin-Induced Gastric Injury in Rats: Targeting DDAH/ADMA and EGFR/ERK Signaling." Scientific Reports vol. 8,1 4280. 9 Mar. 2018, doi:10.1038/s41598-018-22727-6. Distributed under an Open Access license CC BY 3.0, without modification.
For Research Use Only.
