Aristolochic Acid A induced Acute Kidney Injury Modeling & Pharmacodynamics Service
Creative Biolabs offers a range of well-established and robust models for evaluating drug efficacy in AKI, offering customizable solutions to meet your specific research needs. These models allow for detailed assessment of therapeutic agents targeting various aspects of kidney injury and recovery.
Introduction
Acute kidney injury (AKI) is a sudden and severe decline in kidney function, characterized by an abrupt increase in serum creatinine or a decrease in urine output. AKI can occur due to a variety of factors, including ischemia, nephrotoxic drugs, infections, and systemic conditions such as sepsis. The pathophysiology of AKI involves a complex interplay of renal ischemia, inflammation, oxidative stress, and cellular apoptosis, leading to tubular necrosis and impaired kidney function. If left untreated, AKI can progress to chronic kidney disease (CKD) or end-stage renal failure. AKI is associated with high morbidity and mortality rates, making it an urgent medical condition that requires prompt diagnosis and intervention. The disease can affect individuals in various settings, including hospitalized patients, especially those in intensive care units. Early detection and the development of effective therapeutic strategies are critical in improving patient outcomes.
Aristolochic Acid A-Induced Acute Kidney Injury Model
The Aristolochic Acid A-Induced Acute Kidney Injury model is generated by administering AAA to rodents, typically through intraperitoneal or oral dosing. This induces kidney damage resembling the acute stages of human renal failure. The model closely mirrors the clinical presentation of nephrotoxic acute kidney injury seen in patients exposed to certain drugs or herbal remedies. It has been widely used to study the mechanisms underlying kidney injury and to assess the potential therapeutic effects of nephroprotective agents. Despite its reliability, one limitation is the potential for the model to exhibit variations in the severity of damage across subjects, making it essential to control the dosing and monitoring carefully.
- Simulates: This model simulates Aristolochic Acid A-induced nephropathy, including acute tubular necrosis, glomerular damage, and inflammation, which closely mimics the nephrotoxic effects observed in humans.
- Evaluates Drugs: This model is used to evaluate the efficacy of various nephroprotective drugs, including those targeting oxidative stress, inflammatory responses, and renal fibrosis.
Evaluation Platform
- Animals: Mouse, Rat.
-
Measurements
We offer a range of advanced measurements for evaluating drug efficacy in the Aristolochic Acid A-Induced Acute Kidney Injury Model, including:- General Observations: Body weight, survival rate, urine output, and kidney morphology.
- Histopathology: Evaluation of renal tubular necrosis, glomerular damage, and inflammatory cell infiltration via H&E and PAS staining.
- Immunohistochemistry: Detection of kidney injury markers like kidney injury molecule-1 (KIM-1) and inflammatory cytokines.
- Cytokine Profiling: Quantification of pro-inflammatory cytokines (e.g., TNF-α, IL-1β) through ELISA.
- Serum Biomarkers: Blood urea nitrogen (BUN), serum creatinine, and other renal function markers.
- Gene/Protein Expression: RT-qPCR and Western blot analysis for renal fibrosis markers such as TGF-β1, α-SMA, and collagen.
In addition to our standard offerings, our team can customize experiments based on specific research needs and provide consultation throughout the project.
Related Services
We also offer acute kidney injury models induced by other nephrotoxic agents. These alternative models offer varied mechanisms of kidney injury, allowing for more comprehensive drug testing across different pathways of renal dysfunction.
Our advantages
- Expertise in Model Development: Our team has extensive experience in tailoring AKI models for a variety of research needs, ensuring the most relevant and robust findings.
- Comprehensive Data Analysis: We offer in-depth support for experimental design, data collection, and statistical analysis to ensure high-quality results.
- Customizable Protocols: Our models can be adjusted to simulate a wide range of injury severity and therapeutic interventions.
- Cutting-Edge Technologies: We use the latest technologies in histopathology, immunohistochemistry, and gene/protein expression analysis to provide the most accurate and up-to-date data.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What is the significance of using Aristolochic Acid A to induce AKI?
Aristolochic Acid A mimics the nephrotoxic effects commonly seen in patients exposed to certain drugs, making it an ideal model for studying renal failure mechanisms and testing new treatments.
-
2. How long does it take to develop the model?
The model typically requires 24-48 hours after AAA administration to show signs of kidney injury, with peak damage occurring at 72 hours post-treatment.
-
3. What types of drugs can be tested in this model?
The model can evaluate drugs aimed at reducing inflammation, oxidative stress, and fibrosis, as well as those targeting specific kidney injury pathways.
Published Data
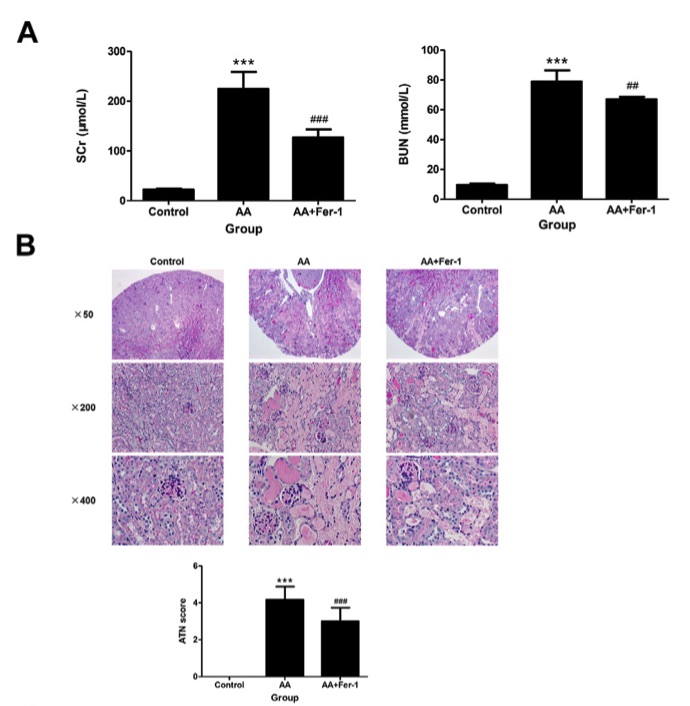 Fig. 1 Renal function and renal histopathology of mice in each group.1
Fig. 1 Renal function and renal histopathology of mice in each group.1
Compared to the control group, mice in the AA group exhibited significantly elevated serum creatinine (SCr) and blood urea nitrogen (BUN) levels, indicating impaired renal function. In contrast, the AA + Fer-1 group showed marked improvements in renal function, with significantly lower SCr and BUN levels compared to the AA group (Figure 1A). Histological examination revealed normal kidney structure in the control group, while kidneys in the AA group exhibited widespread acute tubular necrosis (ATN), characterized by coagulative necrosis, disintegration, and epithelial cell tubular patterns formed by necrotic debris. The tubular structures were dilated, particularly in the deep cortical and corticomedullary regions (Figures 1B, C). The ATN score in the AA group was significantly higher than that of the control group. However, in the AA + Fer-1 group, the ATN score was significantly reduced compared to the AA group (Figure 1B). Electron microscopy revealed that renal tubular epithelial cell mitochondria in the AA group displayed necrotic features, such as swelling, rounding, and severe necrosis with blurred or absent cristae and ruptured outer membranes. In the Fer-1 treatment group, however, no significant changes in mitochondrial morphology were observed (Figure 1D). Overall, the Fer-1-treated group showed milder renal lesions, with focal tubular necrosis, brush border detachment, and cellular vacuolation, presenting a smaller extent of damage compared to the AA group (Figures 1B, C).
Reference
- Huang, Xuan et al. "Aristolochic acid induces acute kidney injury through ferroptosis." Frontiers in Pharmacology vol. 15 1330376. 27 Mar. 2024. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3389/fphar.2024.1330376
For Research Use Only.
