IgA Nephropathy Modeling & Pharmacodynamics Service
Creative Biolabs offers comprehensive services using well-established models for evaluating the therapeutic efficacy of drugs targeting IgA nephropathy. These models allow for in-depth investigation of disease mechanisms, as well as preclinical testing of potential therapies aimed at modulating immune responses and improving renal function.
Introduction
IgA nephropathy (IgAN) is the most common form of primary glomerulonephritis, characterized by the deposition of immunoglobulin A (IgA) in the glomeruli. This immune-mediated disorder primarily affects young adults and can lead to progressive kidney damage, with some patients developing end-stage renal disease. The disease often presents with hematuria, proteinuria, and hypertension, and its pathogenesis involves both genetic and environmental factors. Despite being recognized for its association with increased IgA deposits in kidney tissues, the exact mechanisms driving IgA nephropathy remain poorly understood. Clinically, IgA nephropathy is diagnosed through a kidney biopsy, where mesangial IgA deposition is observed. The progression of the disease varies greatly between individuals, and treatment strategies remain limited.
IgA Nephropathy Model
The IgA nephropathy model is commonly induced in rodents through immunization with bovine serum albumin (BSA) or via genetic modifications that predispose animals to spontaneous IgA deposition. The model replicates key features of human IgAN, including the accumulation of IgA in the glomeruli, renal inflammation, and fibrosis. One of the primary advantages of this model is its ability to mimic the immunological aspects of IgAN, providing insight into the role of IgA in kidney damage. However, variability in disease onset and progression may sometimes limit its reproducibility. This model is widely used to study the immunopathogenesis of IgA nephropathy and to evaluate the efficacy of various therapeutic strategies, including corticosteroids, immunosuppressive agents, and novel biologics aimed at reducing IgA deposition or modifying immune responses.
- Simulates: This model simulates IgA nephropathy, closely mimicking the immune-mediated kidney damage seen in humans, including the deposition of IgA in the glomeruli and the resultant inflammation and fibrosis.
- Evaluates Drugs: It is used to evaluate drugs that target immune responses, reduce glomerular inflammation, and mitigate renal fibrosis, including corticosteroids, immunosuppressive therapies, and investigational biologic agents.
Evaluation Platform
- Animals: Mouse, Rat.
-
Measurements
We offer a variety of measurements for evaluating drug efficacy in IgA nephropathy models, utilizing an array of advanced technologies, including but not limited to:- General observations: Body weight, mortality rate, proteinuria, hematuria, and kidney function assessment (serum creatinine, BUN).
- Immunohistochemistry: Infiltration of immune cells (e.g., T-cells, macrophages) in renal tissues and glomeruli.
- Cytokine profiling (e.g., ELISA): Expression levels of inflammatory mediators such as TNF-α, IL-6, IL-1β, and IFN-γ.
- Renal function markers: Blood urea nitrogen (BUN), creatinine levels, protein-to-creatinine ratio in urine.
- Flow cytometry: Analysis of immune cell populations in kidney tissues and peripheral blood, with a focus on T-cell and B-cell subsets.
- Histopathology: Assessment of glomerular injury, mesangial expansion, and fibrosis in kidney tissues.
In addition to the established IgA nephropathy models, our expertise extends to the development of novel animal models tailored to specific research needs. Our scientific team is available to assist in experimental design, model selection, and data analysis, ensuring a customized and effective approach to your project at every stage.
Related Services
In addition to IgA nephropathy, we also offer other models, which are designed to study the pathophysiology of kidney diseases driven by autoimmune mechanisms. These models help researchers explore new drug candidates targeting autoimmune-related kidney injuries.
- Thy-1 Nephritis Model
- Anti-Glomerular Basement Membrane (GBM) Nephritis Model
- Fx1A Nephritis Model
- Spontaneous Systemic Lupus Erythematosus (SLE) Model
- Systemic Lupus Erythematosus Induced Model
Our advantages
- Tailored Approaches: Our models are adaptable, allowing for specific modifications to simulate various stages and severities of IgA nephropathy.
- High Sensitivity: We employ highly sensitive diagnostic techniques for early detection and accurate assessment of disease progression.
- Expert Consultation: Our team offers continuous support, from model selection to study design and result interpretation.
- Comprehensive Testing: We provide a full spectrum of assays, including histological analysis, flow cytometry, and biomarker profiling, to monitor disease progression and therapeutic responses.
- Strong Track Record: With extensive experience in kidney disease models, we ensure the quality and reliability of our research services.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. How do you induce IgA nephropathy in animal models?
IgA nephropathy can be induced through immunization with bovine serum albumin (BSA) or by using genetically modified rodents predisposed to IgA deposition in the kidneys.
-
2. Can this model be used to evaluate new biologic therapies?
Yes, our IgA nephropathy model is an ideal platform for testing biologic therapies that target immune-mediated kidney damage or reduce IgA deposition.
-
3. What types of biomarkers can be analyzed in this model?
We can analyze a variety of biomarkers, including autoantibodies (e.g., anti-IgA), cytokines (e.g., TNF-α, IL-6), and kidney function markers (e.g., BUN, serum creatinine).
-
4. Is there support for customizing the disease model for specific research needs?
Absolutely. We offer customization options based on your research objectives, including altering the disease severity or targeting specific immune pathways.
-
5. How long does it take to develop the IgA nephropathy model?
The model typically requires 4 to 6 weeks to develop, depending on the induction method and the desired severity of the disease.
Published Data
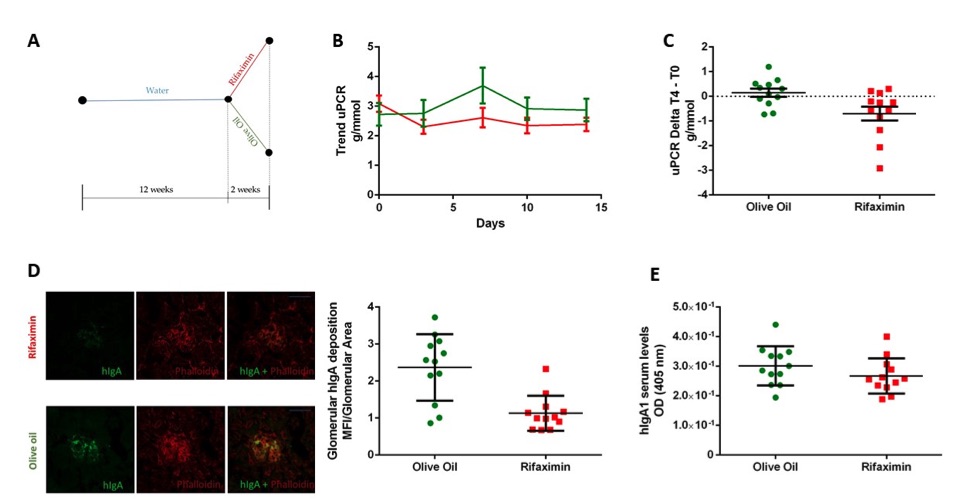 Fig.1 Rifaximin Reduces the Disease Phenotype in IgAN Mice Model.1
Fig.1 Rifaximin Reduces the Disease Phenotype in IgAN Mice Model.1
Twelve-week-old α1KI-CD89Tg mice spontaneously develop mesangial hIgA1 deposition, associated with proteinuria, which mimics IgA nephropathy (IgAN) in humans, as previously described. Mice treated with rifaximin for two weeks showed a reduction in proteinuria, with initial and final uPCR means of 3.09 g/mmol and 2.39 g/mmol, respectively. In contrast, the vehicle group (olive oil) exhibited an increase in proteinuria, with initial and final uPCR means of 2.72 g/mmol and 2.87 g/mmol, respectively. No statistically significant difference in uPCR was observed between the groups at baseline (T0) (Figure 1B), although a significant reduction in uPCR was observed after 14 days of treatment (Figure 1C). Anti-hIgA immunostaining of kidney sections revealed that hIgA1 deposition was significantly reduced in the rifaximin-treated mice compared to the olive oil-treated mice (Figure 1D). Additionally, serum levels of hIgA1, measured by ELISA, were similar in both the rifaximin and vehicle groups (Figure 1E).
Reference
- Di Leo, Vincenzo et al. "Rifaximin as a Potential Treatment for IgA Nephropathy in a Humanized Mice Model." Journal of personalized medicine vol. 11,4 309. 16 Apr. 2021. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3390/jpm11040309
For Research Use Only.
