Monocrotaline induced Pulmonary Hypertension Modeling & Pharmacodynamics Service
Introduction
Hypertension, or high blood pressure, is a significant global health challenge, leading to severe cardiovascular complications, including heart failure, stroke, and kidney disease. pulmonary hypertension (PH), a specific form affecting the arteries of the lungs and the right side of the heart, poses a particularly complex and often life-threatening condition. Effective drug discovery relies on accurate and reproducible preclinical models that closely mimic human disease.
At Creative Biolabs, we are committed to advancing therapeuublished Datatic solutions by providing a comprehensive suite of well-established hypertension models to evaluate drug efficacy rigorously.
Monocrotaline-Induced PH Model
The monocrotaline (MCT)-induced PH model is a widely recognized and extensively used preclinical model for studying pulmonary vascular disease and evaluating potential therapeutic agents. This model reliably induces a form of PH characterized by significant pulmonary vascular remodeling and subsequent right ventricular hypertrophy, closely mirroring key pathological features observed in human pulmonary arterial hypertension (PAH).
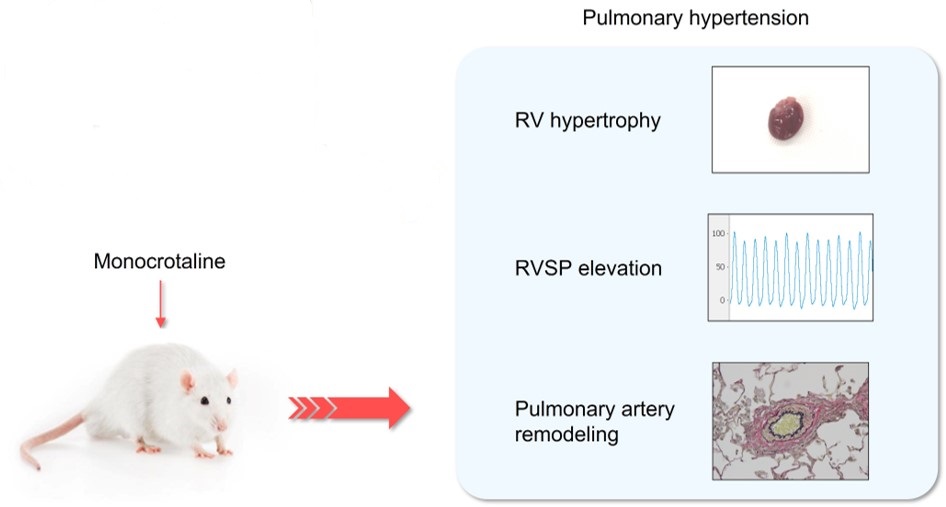 Fig.1 Experimental model of MCT-induced PH.1
Fig.1 Experimental model of MCT-induced PH.1
Model Construction Steps
The construction of the MCT-induced PH model typically involves a single administration of MCT to rodents, most commonly rats, to initiate pulmonary vascular injury. This standardized approach ensures high reproducibility of the disease phenotype.
01Animal Selection
Healthy, age-matched rodents are selected, typically weighing between 150-250 grams.
02MCT Preparation
MCT is dissolved in a suitable vehicle (e.g., 0.1 N HCl, adjusted to pH 7.4 with NaOH) immediately before administration.
03Administration
A single dose of MCT (e.g., 60 mg/kg) is administered via subcutaneous or intraperitoneal injection.
04Observation Period
Animals are monitored for 3-4 weeks, during which PH develops progressively. Body weight, general health, and signs of distress are regularly assessed.
05Endpoint Analysis
At the study's conclusion, comprehensive phenotyping is performed, including hemodynamic measurements, histological analysis of lung and heart tissues, and molecular assessments.
Strengths and Limitations
Strengths:
- High Reproducibility: Offers consistent induction of PH, facilitating reliable drug efficacy studies.
- Cost-Effective: Generally more economical and less technically demanding than genetic or surgical models.
- Well-Characterized Pathology: Closely mimics key features of human PH, including vascular remodeling and right heart hypertrophy.
- Extensive Historical Data: Supported by decades of research, providing a robust foundation for study design and interpretation.
Limitations:
- Acute Disease Onset: The rapid development of PH may not fully represent the chronic progression of all human PH subtypes.
- Lack of Plexiform Lesions: Does not typically develop the complex plexiform lesions characteristic of severe human idiopathic PAH.
- Species-Specific Responses: Potential differences in drug metabolism and disease progression can exist across species.
Evaluation Platform
Creative Biolabs provides a state-of-the-art evaluation platform for a comprehensive assessment of the MCT-induced PH model. Our capabilities and key test indicators include:
- Comprehensive Assessment: Spanning biochemical, molecular, cellular, histopathological, behavioral, and advanced imaging techniques.
- Hemodynamic Parameters: Direct measurement of right ventricular systolic pressure (RVSP) and mean pulmonary arterial pressure (mPAP).
- Cardiac Remodeling: Quantification of right ventricular hypertrophy using the Fulton index (RV/(LV+S)).
- Pulmonary Vascular Remodeling: Detailed analysis of medial thickness and muscularization of pulmonary arteries.
- Inflammatory Markers: Assessment of relevant inflammatory mediators.
- Functional Capacity: Evaluation of exercise capacity and other behavioral indicators.
Applications
- Modeling Disease Pathogenesis: This model reliably simulates key aspects of human PAH (Group 1 PAH) and PH due to lung disease/hypoxia (Group 3 PH), particularly focusing on the crucial processes of pulmonary vascular remodeling.
- Evaluating Drug Efficacy: It serves as an ideal platform for assessing the therapeutic potential of novel compounds, including vasodilators, anti-proliferative agents, anti-fibrotic compounds, anti-inflammatory drugs, and agents specifically targeting right ventricular function.
- Assessing Novel Therapeutic Approaches: Beyond traditional small molecules, the model is well-suited for evaluating advanced treatment modalities such as biologics, gene therapies, and cell-based therapies, providing insights into their efficacy in mitigating PH.
- Investigating Disease Mechanisms: The model provides a robust and controlled environment to delve into the underlying molecular and cellular mechanisms that drive PH progression and, conversely, those that contribute to disease regression or reversal.
- Discovering and Validating Biomarkers: It is an invaluable tool for identifying and validating novel biomarkers that can serve as indicators for disease diagnosis, monitor progression, predict prognosis, or assess the response to therapeutic interventions.
Related Hypertension Models
Our Advantages
- Deep Expertise: Years of specialized experience in preclinical PH research and model development.
- Comprehensive Phenotyping: Full suite of gold-standard and advanced assays for precise and reliable data.
- Customized Study Design: Flexible protocols tailored to your specific research objectives and compound characteristics.
- Rapid Turnaround: Efficient project management ensures timely delivery of high-quality, actionable data.
- Ethical Compliance: Strict adherence to international animal welfare guidelines and ethical research practices.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
Contact Us
Creative Biolabs is dedicated to advancing PH research. We offer comprehensive services utilizing the MCT-induced PH model, backed by extensive experience and cutting-edge capabilities. We invite you to connect with our scientific team to discuss your specific project needs and explore how we can support your drug discovery efforts.
FAQs
-
Q1: Can this model be used to evaluate both preventive and therapeutic interventions?
A: Absolutely. The MCT-induced PH model is highly versatile and can be effectively utilized for both preventive and therapeutic studies. For preventive studies, the test compound is administered concurrently with or shortly after MCT. In therapeutic studies, the compound administration begins after PH has been established, allowing for the evaluation of its ability to reverse or halt disease progression.
-
Q2: What are the key histological endpoints evaluated in lung tissue from MCT-treated animals?
A: Histological evaluation of lung tissue is crucial for assessing pulmonary vascular remodeling. Key endpoints include quantifying the medial wall thickness of pulmonary arteries, assessing the degree of muscularization in previously non-muscularized arterioles, and evaluating the extent of lumen narrowing. We also look for signs of perivascular inflammation and fibrosis, which are characteristic features of the disease.
-
Q3: How does Creative Biolabs ensure the reproducibility of the MCT-induced PH model?
A: Creative Biolabs ensures high reproducibility through rigorously standardized protocols, including precise MCT dosing and administration routes. We utilize genetically consistent animal strains and maintain strict environmental controls. Our experienced team performs meticulous animal monitoring and applies standardized endpoint assessments, including comprehensive quality control measures for all data generated.
-
Q4: What are the common challenges encountered when working with the MCT model, and how do you address them?
A: Common challenges include inter-animal variability in disease severity and potential systemic toxicity of MCT. We address these by optimizing dosing regimens, implementing strict animal health monitoring, and employing robust statistical analysis. Our extensive experience allows us to fine-tune protocols to minimize variability and maximize the scientific validity of the results.
-
Q5: Can you customize the MCT model to fit specific research needs, such as combination therapies or different disease stages?
A: Yes, customization is a core strength of Creative Biolabs. We can tailor the MCT model to accommodate various research needs, including evaluating combination therapies, assessing compounds at different stages of PH progression (preventive vs. therapeutic), and integrating specific biomarkers or advanced imaging techniques. Our scientific team works closely with clients to design bespoke study protocols.
Published Data
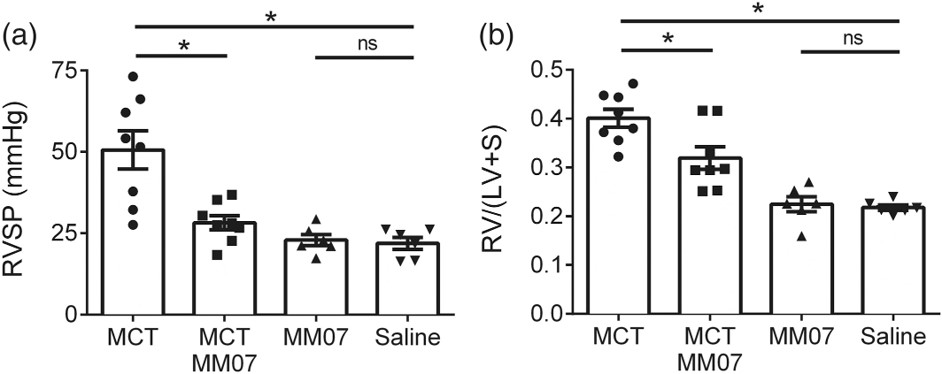 Fig.2 Attenuation of MCT-induced PAH by MM07 administration.2
Fig.2 Attenuation of MCT-induced PAH by MM07 administration.2
A compelling study provides a strong example of the model's utility in evaluating novel therapeutic agents. This article investigated a G protein-biased apelin analogue, MM07. The project results demonstrated that MM07 significantly reduced the elevation of right ventricular systolic pressure and hypertrophy, attenuated MCT-induced changes in cardiac structure and function, and significantly reduced pulmonary vascular muscularization, highlighting its disease-modifying potential in PH.
References
- Tawa, Masashi, et al. "Ameliorative effects of beetroot juice supplementation on monocrotaline-induced pulmonary hypertension in rats." Future Pharmacology 2.4 (2022): 547-557. Distributed under Open Access license CC BY 4.0. The image was modified by extracting and using only part of the original image. https://doi.org/10.3390/futurepharmacol2040033
- Yang, Peiran et al. "A novel cyclic biased agonist of the apelin receptor, MM07, is disease modifying in the rat monocrotaline model of pulmonary arterial hypertension." British journal of pharmacology vol. 176,9 (2019): 1206-1221. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1111/bph.14603
For Research Use Only.
