Cerulein induced Acute Pancreatitis Modeling & Pharmacodynamics Service
Introduction
Acute Pancreatitis is a sudden inflammation of the pancreas characterized by activation of digestive enzymes within the pancreas itself, leading to autodigestion, tissue damage, and inflammation. It is a potentially life-threatening condition with a wide clinical spectrum ranging from mild, self-limiting disease to severe forms with necrosis, organ failure, and high mortality. The most common causes are gallstones and chronic alcohol consumption, though metabolic disorders, certain medications, infections, and trauma can also trigger the condition. Acute pancreatitis is broadly categorized into two types: interstitial edematous pancreatitis, which is more common and involves inflammation without significant tissue death, and necrotizing pancreatitis, which involves pancreatic tissue necrosis and carries a higher risk of complications. Patients typically present with sudden upper abdominal pain, elevated serum amylase and lipase levels, and systemic inflammatory symptoms. Despite advances in care, no targeted pharmacological therapies currently exist, and treatment remains largely supportive. Creative Biolabs offers a range of well-established animal models to evaluate drug efficacy for acute pancreatitis, including cerulein-induced, L-arginine-induced, and sodium taurocholate-induced models. Our services include model customization, biomarker analysis, histological evaluation, and study design support.
Disease Models and Applications
The Cerulein-Induced Acute Pancreatitis Model is one of the most widely used rodent models for studying acute pancreatitis. This model is established by administering supramaximal doses of cerulein—an analog of the digestive hormone cholecystokinin—via intraperitoneal injections, typically given hourly over a 6–10 hour period. Cerulein overstimulates pancreatic acinar cells, leading to the activation of trypsinogen, enzyme autodigestion, edema, and inflammatory cell infiltration. This model replicates many clinical and histological features of human mild to moderate acute pancreatitis. Advantages include its reproducibility, ease of induction, and suitability for studying early inflammatory responses. However, it typically does not replicate severe necrotizing pancreatitis and systemic complications, limiting its translational relevance in such cases.
Simulates: The Cerulein-Induced Acute Pancreatitis Model simulates mild to moderate interstitial edematous pancreatitis observed in humans. It effectively mimics pancreatic inflammation, enzyme activation, cytokine release, and tissue edema.
Evaluates Drugs: This model is widely used to evaluate anti-inflammatory drugs, protease inhibitors, immunomodulators, cytokine blockers (e.g., IL-1β or TNF-α inhibitors), antioxidants, and agents targeting oxidative stress and apoptosis in pancreatic tissues.
Measurements
We offer a comprehensive set of measurements specifically tailored for the Cerulein-Induced Acute Pancreatitis Model to assess drug efficacy and pathophysiological changes:
- General Observations: Body weight change, behavior, mortality rate, and signs of abdominal distress.
- Pancreatic Histopathology: Edema, inflammatory cell infiltration, acinar cell vacuolization, and necrosis scores.
- Serum Biomarkers: Elevated levels of amylase and lipase as key indicators of pancreatic injury.
- Cytokine Quantification (ELISA): TNF-α, IL-1β, IL-6 levels in serum and pancreatic tissue homogenates.
- Myeloperoxidase (MPO) Activity: As a marker for neutrophil infiltration.
- RT-qPCR and Western Blot: Expression of inflammatory markers, apoptosis-related genes (e.g., Bcl-2, Bax), and endoplasmic reticulum stress-related proteins.
- Immunohistochemistry: Localization and quantification of macrophages, T-cells, and apoptosis markers within pancreatic tissue.
In addition to established models, we specialize in developing tailored animal models based on published protocols and your unique research objectives. Our experienced team supports study design, model selection, and comprehensive data analysis to ensure precision and reliability.
Related Services
In addition to the acute pancreatitis model, we provide various alternative induction methods to meet diverse research needs. Our platform ensures flexibility and reliability.
- Sodium Taurocholate induced Acute Pancreatitis Model
- Caerulein & LPS induced Acute Pancreatitis Model
Advantages
- Comprehensive Expertise: Our team has extensive experience in modeling acute pancreatitis and other gastrointestinal diseases, ensuring scientifically sound and reproducible results.
- Diverse Model Portfolio: We offer multiple validated models, including cerulein-, L-arginine-, sodium taurocholate-, and alcohol-induced pancreatitis, to simulate different disease severities and mechanisms.
- Advanced Technologies: Equipped with cutting-edge tools for histology, molecular analysis, imaging, and biomarker quantification, we deliver high-quality and multidimensional data.
- Customized Study Design: We tailor protocols to align with your therapeutic goals, including dosing schedules, endpoints, and specialized assays.
- End-to-End Support: From experimental planning to final reporting, our dedicated scientific and technical teams provide seamless project execution and transparent communication.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q1: What species do you use for cerulein-induced AP models?
A1: We typically use C57BL/6 mice or Sprague-Dawley rats, depending on the study requirements.
-
Q2: Can this model be used to test biologics or gene therapies?
A2: Yes, the model is suitable for evaluating a wide range of therapeutics, including biologics, peptides, and gene-editing approaches.
-
Q3: What is the typical timeline for an AP study?
A3: From induction to final sample collection, studies usually take 1–2 weeks, depending on your design.
-
Q4: Can we request additional tissues or downstream analyses?
A4: Absolutely. We offer customizable tissue collection, omics analysis, and biomarker assays upon request.
Published Data
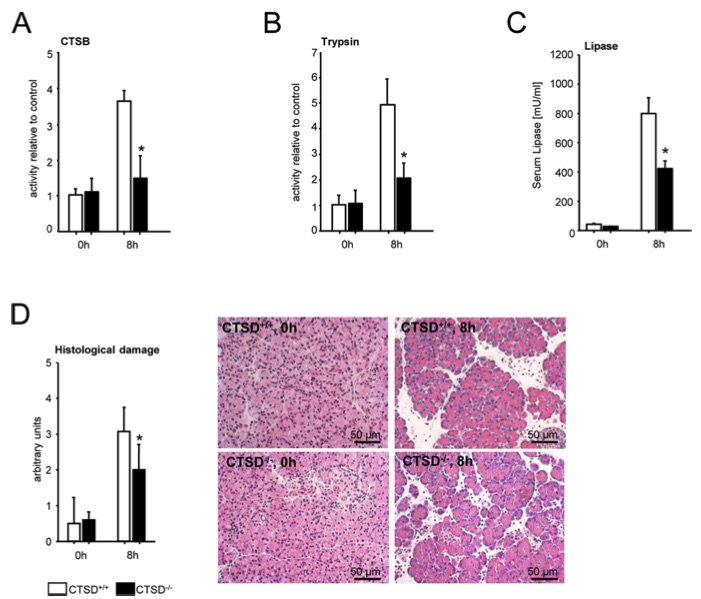 Fig.1 Caerulein-induced acute pancreatitis in CTSD-/- mice (complete knockout) and littermate controls.1
Fig.1 Caerulein-induced acute pancreatitis in CTSD-/- mice (complete knockout) and littermate controls.1
To investigate the role of cathepsin D (CTSD) in modulating the severity of acute pancreatitis, this article conducted an in vivo study using the cerulein-induced acute pancreatitis model. CTSD knockout (CTSD⁻/⁻) mice and wild-type C57BL/6 controls were utilized at 14 days of age to avoid the confounding effects of intestinal atrophy and lymphoid tissue loss seen in older animals. The study focused on the late phase of pancreatitis, assessed at 8 hours post-induction. Unlike acinar cell-specific CTSD deletion, global CTSD deficiency—affecting both acinar and inflammatory cells—led to a marked reduction in cathepsin B (CTSB) activation (Fig. 1A), trypsin activation (Fig. 1B), serum lipase levels (Fig. 1C), and cumulative histopathological damage (Fig. 1D). These findings indicate that while the CTSD–CTSB–trypsin cascade in acinar cells plays a limited and transient role, CTSD primarily contributes to the severity of pancreatitis through its function in non-acinar cell types.
Reference
- Aghdassi, Ali A et al. "Cathepsin D regulates cathepsin B activation and disease severity predominantly in inflammatory cells during experimental pancreatitis." The Journal of Biological Chemistry vol. 293,3 (2018): 1018-1029. DOI:10.1074/jbc.M117.814772. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
